Method Article
Characterization of Sickling During Controlled Automated Deoxygenation with Oxygen Gradient Ektacytometry
In This Article
Summary
Here, we present oxygen gradient ektacytometry, a rapid and reproducible method to measure red blood cell deformability in samples from patients with sickle cell disease under controlled deoxygenation and reoxygenation. This technique provides a way to study red blood cell sickling and to monitor sickle cell disease treatment efficacy.
Abstract
In sickle cell disease (SCD), a single point mutation in the gene coding for beta-globin causes the production of abnormal hemoglobin S (HbS). When deoxygenated, HbS can polymerize, forming rigid rods of hemoglobin, resulting in the sickling of red blood cells (RBCs). These sickled RBCs have significantly reduced deformability, causing vaso-occlusion, which leads to numerous SCD-related clinical complications, including pain, stroke, and organ damage. RBC deformability is also reduced by RBC dehydration, resulting in dense red blood cells that are more likely to sickle. To date, there is not a single widely available, rapid, and reproducible laboratory assay capable of predicting the disease severity or directly monitoring the treatment effects for novel, non-fetal hemoglobin inducing therapies. In this study, we describe a protocol to measure RBC deformability as a function of pO2 that allows for the quantitation of sickling behavior in SCD patients. Oxygen gradient ektacytometry measures RBC deformability, expressed as the elongation index (EI), as a function of pO2. RBCs are exposed to a fixed shear stress of 30 Pa during one round of deoxygenation and reoxygenation. Six readout parameters are produced. Of these, the point of sickling (PoS), defined as the pO2 at which maximum EI (EImax) shows a 5% decrease, and minimum EI during deoxygenation (EImin) are the most informative, reflecting an individual patient’s pO2 at which sickling starts and the minimal deformability of a patient’s red blood cells, respectively. PoS is associated with an individual patient’s hemoglobin affinity for oxygen, whereas EImin shows a strong correlation with fetal hemoglobin levels. We conclude that oxygen gradient ektacytometry is a promising technique to monitor the treatment of patients with SCD, as a biomarker for anti-sickling agents in clinical and preclinical trials, and an important tool to study sickling behavior of RBCs from individuals with SCD and sickle cell traits.
Introduction
In SCD, a single point mutation results in the production of HbS, which can polymerize upon deoxygenation. HbS polymerization causes sickling of RBCs and reduces RBC deformability. The combination of RBC sickling and RBC adherence to the endothelium leads to various SCD complications, including vaso-occlusive crises (VOC), stroke, organ damage, and chronic hemolytic anemia. Even at normoxic conditions, RBC deformability is compromised in patients with SCD. Deformability is further decreased at low oxygen concentrations. Key players that determine deformability at normoxia are dense cells, irreversibly sickled cells (ISC), and dehydrated cells, all of which have a decreased surface-to-volume ratio1,2,3.
Ektacytometry is an established method to measure RBC deformability, widely used for the diagnosis of hereditary hemolytic anemias, particularly membranopathies4. It can also be used to study hemorheology5,6,7,8,9. Osmotic gradient ektacytometry, in which RBC deformability is measured during a continuous change in osmolality, has been used to study SCD for over a decade10,11. The percentage of fetal hemoglobin (HbF) is one of the strongest inhibitors of HbS polymerization because neither HbF nor its mixed hybrid tetramer (∝2βSγ) can enter the deoxyHbS polymer phase12. Recent studies suggest that increasing HbF levels in SCD patients leads to a better surface-to-volume ratio, thereby improving the hydration state and thus the deformability in nontransfused patients11.
RBC deformability has been studied in the past as a biomarker for SCD complications, but with conflicting results. In studies performed cross-sectionally and at a steady state, individuals with higher levels of RBC deformability were found to have a higher incidence of osteonecrosis and more pain crises13,14,15. In contrast to these findings, when compared to the steady state values during an acute VOC, RBC deformability was decreased in longitudinal studies within the same individuals16. This discrepancy may be the result of studying RBC deformability under different conditions (i.e., during the steady state versus VOC). The percentage of sickled cells is high at the start of a VOC and the cells are rapidly destroyed as the crisis progresses, which may explain the difference between the steady state cross-sectional incidence data and longitudinal data obtained during the VOC. However, other factors, such as adherence of RBC subpopulations to the endothelial surface, may also be important in the occurrence of VOC. In SCD, it is more clinically relevant to measure the deformability during the deoxygenation, because vaso-occlusion typically occurs in the hypoxic postcapillary venules and not in the less hypoxic microcapillary network17. Additionally, the presence of ISCs may alter the ability of an ektacytometer to measure the deformability at normoxia. Distortion of the diffraction pattern is caused by ISCs, which results from the non-alignment during the flow1,2,3.
Alternative approaches to study the pathophysiology of VOC include measurements of RBC adherence to an artificial surface18, single cell electrical impedance microflow cytometry19, microfluidic-based models combining quantitative measurements of the cell sickling and unsickling with single cell rheology20, and laser-induced polymerization21. Although promising, these techniques are costly, labor intensive, and require extensive operator training. In addition, the assays that are morphology-based lack the ability to study cellular behavior, such as deformability, as a function of an oxygen gradient.
In this study, we describe a rapid and reproducible functional assay performed with an ektacytometer. This is a next generation ektacytometry measurement that measures the different qualitative aspects of RBC deformability expressed as the EI during deoxygenation (1,300 s) and swift reoxygenation (280 s). These time intervals allow for HbS polymer formation, and thereby the occurrence of morphological changes and then recovery. Deoxygenation occurs by introducing nitrogen gas, which slowly decreases the oxygen tension in the blood sample in the gap between the bob and cup of the ektacytometer. RBC deformability is continuously measured while oxygen tension is measured every 20 s by means of a small O2-spot present in the wall of the cup. During the test, approximately 80 pO2 measurements are coupled to the EI measured at that moment. The oxygen pressure drops below 20 mmHg during the deoxygenation, and reoxygenation is facilitated by the passive diffusion of ambient air. The experimental setup of the ektacytometer and oxygen gradient ektacytometry module is described in Figure 1 and Figure 2. The principle of ektacytometry is based on RBC-induced scattering of light from a laser beam. This results in an elliptical diffraction pattern when shear stress is applied at the same time (Figure 1).
Protocol
All procedures were approved by the ethical committee of the University Medical Center Utrecht (UMCU) and in accordance to the Declaration of Helsinki. Patients enrolled at the Texas Children’s Hematology Center (TCHC) were approved by the local IRB and in accordance with the Declaration of Helsinki.
1. General considerations
- Begin by performing a test measurement to warm up the bob and cup. Ensure that the temperature of the bob and cup is 37 ˚C. This is important for good reproducibility.
- Ensure that the viscous polyvinylpyrrolidone (PVP) solution falls within the strict limits for osmolarity (282–286 mOsm/kg), pH (7.35–7.45) and viscosity (27.5–32.5 MPa) at room temperature (22 ˚C).
NOTE: The PVP must be used at room temperature. If stored at a lower temperature, make sure it has warmed up to room temperature prior to taking any measurements.
2. Start-up of the ektacytometer
- Switch on the computer and the ektacytometer from the back. Start the software program (Table of Materials) on the computer.
- Make sure the nitrogen is available to deoxygenate the sample by opening the nitrogen cylinder.
- Lower the bob in the cup and make sure the cup can turn freely. Clean the cup on the inside and outside with a soft cloth and distilled water, because debris can hamper the EI measurements.
- When the software program is running, check for the following message on the screen: "Make sure the gas valve is open" and click OK.
- Ensure that the ektacytometer starts the pO2 self-check process that will appear on the screen. Select Start (enter). If it fails, rerun the self-check by clicking Hardware check | pO2 | Self check.
NOTE: If the self-check fails again, consider replacing the O2-spot. The O2-spot is replaced by gently pushing the spot out from the inside of the cup with a fingertip. A new spot is placed by gently pushing the spot from the outside into the cup. - Choose pO2 scan from the different tests listed on the left. Choose Settings at the right of the screen and ensure they are set as per the parameters listed in Table 1. Keep the same settings for every measurement.
- In order to save these settings, press OK | OK.
NOTE: Preferred settings are listed in Table 1 but can be adjusted according to the user preferences and investigational purposes. For example, to study the sickling behavior more extensively, deoxygenation speed and duration can be altered.
3. Sample collection and preparation
NOTE: For the validation of the technique, ethylenediamine tetraacetic acid (EDTA)-treated blood from 38 SCD patients and 5 healthy controls included at the University Medical Center Utrecht or Texas Children's Hematology Center, in different clinical studies (Netherlands Trial Registry [NTR] identifier, NTR 6779 and NTR 6462), as well as anonymized leftover blood samples from patients who visited the outpatient clinic or were hospitalized were used.
- Collect blood samples by venipuncture (a minimum of 300 µL/sample) in a tube containing EDTA. Make sure the blood has been stored for at least 30 min at 4 °C, but no longer than 24 h.
NOTE: Citrate phosphate dextrose adenine (CPDA) or heparin can also be used, but the influence of these reagents on the sample preservation with respect to the oxygen gradient ektacytometry is not well-known. - Mix the sample gently by inversion to homogenize. Do not shake the sample. Let the sample warm up to room temperature on a roller bench before the measurement.
NOTE: A sample tube (9–10 mL) that is stored for more than 1 h at 4 ˚C must warm up for 15 min. When stored for less than 1 h at 4 ˚C, it must warm up for 10 min. A sample tube (2–6 mL) that is stored for more than 1 h at 4 ˚C must warm up for 10 min. When stored for less than 1 h at 4 ˚C, it must warm up for 5 min. - Measure the complete blood count on a hematology analyzer. To do so, take 20–200 µL of whole blood in a tube containing EDTA. Place the aspiration needle in the tube and press on the button behind the needle of the hematology analyzer to start the measurement.
NOTE: In the complete blood count, the RBC number is measured, which is an important factor for standardizing the oxygen gradient ektacytometry measurements. RBC count is calculated from forward and sideward scatter by flow cytometry. Normal RBC count in healthy controls is 3.7–5.0 x 1012/L for females and 4.2–5.5 x 1012/L for males. RBC count in patients with SCD is generally decreased. Some hematology analyzers will also measure percent dense red blood cells (% DRBC) which can be of additional value in the interpretation of individual oxygen gradient ektacytometry curves. - Standardize the whole blood sample to an RBC count of 200 x 106 RBCs in 5 mL PVP (200 x 106 RBCs/vial) by adjusting the volume of sample that will be added. If the total RBC count is less than 200 x 106, the diffraction pattern and EI will be affected.
- Use the equation below to perform the counting.
4.0/xx (x 1012/L) x 50 = yy µL whole blood/vial PVP
where xx is the calculated RBC count obtained from step 3.3 and yy is the amount of whole blood that is required for the actual measurement. Depending on the grade of anemia and other factors influencing RBC counts, the amount of whole blood required is 40–90 µL.
- Use the equation below to perform the counting.
4. Oxygen gradient ektacytometry measurement
- Pipette the calculated sample volume (yy µL of blood) into PVP to obtain a total volume of 5 mL. Prewet the tip by gently resuspending the blood 3x. Use a pipette tip with a wide opening to avoid additional stress on the RBCs. Gently mix the sample manually by inversion until it is homogeneous.
NOTE: Open the PVP vial for as short a time as possible to avoid air contact. - Slowly draw 2.0 mL of the blood/PVP mixture into a 3 mL syringe without the needle. Push the plunger to remove any visible air bubbles and excessive sample solution until 1.5–1.8 mL is left in the syringe (depending on the cup volume).
- Inject the total sample volume slowly and evenly in the bob through the connector. Make sure the level of the sample is above the oxygen sensor (pink spot) and above the small suction hole. Do not leave any sample solution in the syringe.
- Click New and fill in the sample identifier, remarks, date of donation, and viscosity of PVP. Click OK |Aspirate. After 60 s, the cup will rotate and aspirate the sample for 15 s. Click OK when the rotation stops. Close the machine lid. Click Continue | Start now, as oxygen gradient ektacytometry is done with a fixed gain. The measurement will take about 28 min.
- After the measurement, print the report that shows the curve and parameters that are automatically calculated by the software. Ensure that the raw data is automatically stored in the designated folder in Settings. Maximum EI (EImax), minimum EI (EImin), pO2@95%EI (PoS), and area (area under the curve) are automatically calculated and added to the printed report and raw data.
- Manually obtain ΔEI by calculating the difference between EImax and EImin. Calculate the percentage recovery by taking the difference in mean EI before deoxygenation (pO2 100–120 mmHg) and mean EI values during reoxygenation at 100–120 mmHg.
5. Cleaning of the ektacytometer
- Remove the sample syringe and replace it with a syringe filled with distilled water or deionized water.
- Press Clean, slowly flushing the connector during rinsing. Make sure to flush in both directions.
- Remove the syringe and lift the bob. Dry the bob, cup, and connector thoroughly with a soft cloth.
- Use a large syringe (10–50 mL) to flush the connector in order to remove any water remaining in the tubes and bob. Block the lower inlet/outlet of the bob to get back pressure in the tubes, thereby removing remaining water.
- Lower the bob in the cup. The machine is now ready for the next measurement.
6. Shutdown of the machine
- Ensure the machine is properly rinsed after the last measurement, as described above. Ensure the proper tubes relate to the cleaning solution.
- Close the software, press Close, and press Start to start end-of-day cleaning program.
- After completing the whole cleaning program, remove the syringe and lift the bob. Flush the connector with a big syringe.
- Empty the waste bottle and dry the bob and cup with a soft cloth. Flush the connector in order to remove the water remaining in the tubes and bob. Block the lower inlet/outlet of the bob to get back pressure in the tubes, thereby removing any remaining water.
- Close the lid of the machine. Close the nitrogen cylinder. Turn off the computer and the machine.
Results
Oxygen gradient ektacytometry can be used to characterize sickling behavior in patients with SCD. In this study, blood samples from a total of 38 SCD patients and five healthy controls were included. In healthy controls, the diffraction pattern is circular at rest and elliptical at higher shear stress4. From the elliptical diffraction pattern, the elongation index (EI) is calculated based on the height and width of the diffraction pattern. In oxygen gradient ektacytometry, slow and continuous deoxygenation of the sample by nitrogen gas is followed by swift reoxygenation by ambient air. Under these conditions, RBC sickling can be observed under deoxygenation. This will cause a distortion of the diffraction pattern because sickled red cells will not align properly under the applied shear stress. Hence, they appear to be less deformable as opposed to healthy RBCs (Figure 2).
Figure 3A shows how sickle RBCs change in shape upon deoxygenation, which mimicked conditions during oxygen gradient ektacytometry, whereas control sickle RBCs without deoxygenation show no change in shape. This process results in distortion of the diffraction pattern during oxygen gradient ektacytometry, and thus in a decrease in EI. Figure 3B shows the different diffraction patterns from which different parameters are generated.
A representative curve obtained by the ektacytometer is shown in Figure 3C. Six parameters reflect different characteristics of sickling behavior of RBCs: EImax is the maximum EI at the start of the measurement before deoxygenation. This parameter represents the baseline position and reflects the overall deformability of the total RBC population at ambient air. EImin is the minimum EI, which represents minimal deformability after deoxygenation. This parameter reflects changes in the shape and orientation of (sickle) RBCs upon deoxygenation. ΔEI is the difference between EImax and EImin, which indicates how many cells can sickle during one round of deoxygenation. 5% Point of Sickling (PoS5%) is the pO2 (mmHg) at which a 5% decrease of EImax during deoxygenation is measured. This represents the oxygen tension where the sickling process starts. Area reflects the area under the curve, which is determined by an integral calculation of EI and pO2 measurements between 100 mmHg and pO2min (mmHg). This is the result of previously described parameters EImax, EImin, and PoS. Recovery represents the difference of EI during the final part of reoxygenation compared to EI at baseline. Both EI values are measured at a pO2 of 100–120 mmHg. This parameter reflects the capacity of RBCs that sickle during deoxygenation to reverse sickling during reoxygenation22. Parameters from duplicate measurements generally had a coefficient of variation (CV) <5% (median 1.83%). In case a CV > 5% was obtained, a third measurement was performed. The parameters EImax and Recovery are the most reproducible with median CVs <1%.
Representative curves of RBCs of healthy controls, patients with HbS traits (heterozygous HbS), and a homozygous SCD patient are shown in Figure 4A. The representative curve of the HbSC patient shows a lower recovery, which might indicate a different sickling process (Figure 4B). The representative curves of HbSS patients treated with hydroxyurea (HU) and transfusion are shown in Figure 4C and Figure 4D. Clearly, there is a big difference between the representative curves of HS traits (HbAS cells) and RBCs of HbSS patients treated with transfusion (consisting of a mixture of homozygous sickle (HbSS) and homozygous normal (HbAA) cells, Figure 4A,D). The clear differences in the curves of the untreated SCD patient and the HU and transfusion-treated patients highlights the usefulness of this assay (Figure 4C,D). Levels of HbF and HbS correlated significantly with EImin and, to a lesser extent, with PoS (Figure 5A–D). This indicates that those laboratory parameters that are important in the evaluation of the patient are also reflected in the oxygen gradient ektacytometry. The number of sickled cells at normoxia and percentage of dense RBCs (DRBCs) both influence EImax values, as they are significantly correlated (Figure 5E–F), which indicates that EImax reflects another important factor in the sickling process. These results show how different characteristics such has %HbS, %HbF, sickled cells at normoxia, and %DRBCs influence different parameters.
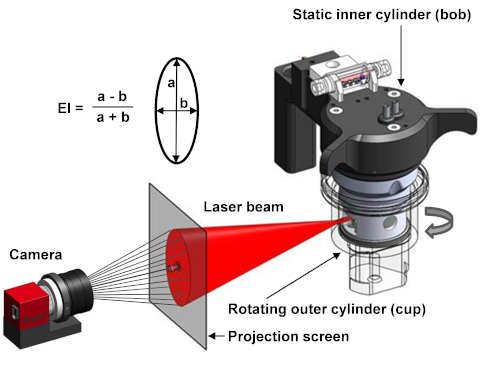
Figure 1. Schematic setup of the ektacytometer. The ektacytometer uses a Couette system to apply shear stress on the cells. A rotation outer cylinder (cup) and a static inner cylinder (bob) are used to induce shear stress by the creation of laminar flow at 37 ˚C. Between the bob and cup there is a small gap in which the blood suspension is injected. A laser beam shines from the bob through the blood suspension and is scattered by the presence of RBCs. The diffraction pattern is projected and analyzed by a camera. The elongation index (EI) is calculated with the height (a) and the width (b) of the diffraction pattern4. Please click here to view a larger version of this figure.
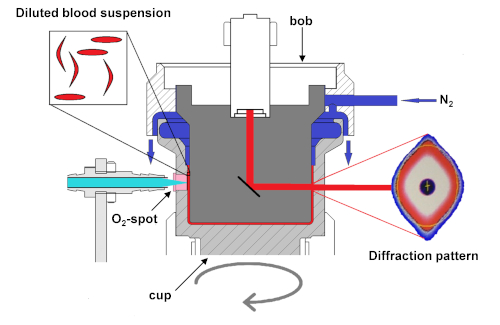
Figure 2. Schematic setup of the ektacytometer with oxygen gradient ektacytometry module. Schematic diagram of the module that shows deoxygenation of the blood suspension slowly with the infusion of nitrogen gas (N2). Oxygen tension is measured by the amount of quenching of the luminophore signal sent from the LED-fiber to the O2-spot. Upon deoxygenation, sickle RBCs will start to sickle, their deformability will decrease, and they will no longer align with elliptical RBCs. The sickled RBCs will distort the diffraction pattern, changing its shape from an ellipse to a rhomboid or diamond-like shape. This change in the shape of the diffraction pattern results in a decrease of EI. Measurements of pO2 and EI are not performed at the same height in the cup. This ensures better discrimination between the deoxygenation and reoxygenation curves and, hence, a better interpretation of the curve. This figure has been modified from Rab et al.22 Please click here to view a larger version of this figure.
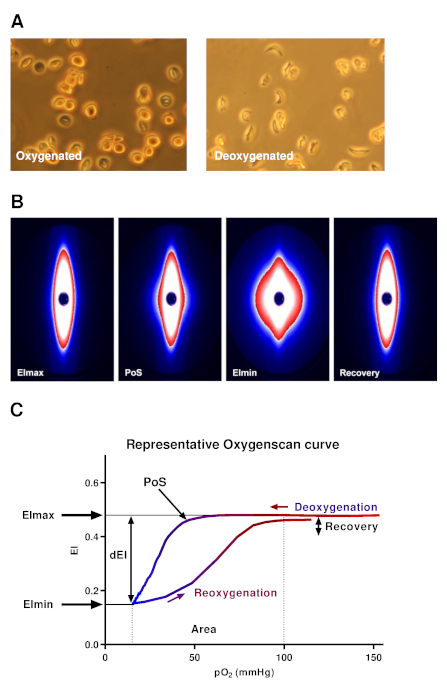
Figure 3. Representative oxygen gradient ektacytometry curve and diffraction patterns. (A) Upon deoxygenation under conditions similar to the oxygen gradient ektacytometry, sickle RBCs were fixed. In control sickle RBCs, the same conditions were used, but without nitrogen gas. Deoxygenated sickle RBCs show a change in shape in contrast to control RBCs. (B) Upon deoxygenation and shear stress (30 Pa), the diffraction pattern changes from an ellipse to a rhomboid. (C) Representative curve of oxygen gradient ektacytometry. The maximum elongation index (EImax) represents the baseline position and shows an overall deformability of the total RBC population. Minimum EI (EImin) represents minimal deformability, which is caused by the change in shape and orientation of RBCs upon deoxygenation. ΔEI (dEI, the difference in EI between EImax and EImin) shows how many cells can sickle during one round of deoxygenation. Point of sickling (PoS, pO2 at 5% EI decrease) shows the oxygen tension when the first RBCs start to sickle. The area under the curve (from pO2min = 100 mmHg) is calculated in the parameter area. This summarizes EImax, EImin, and PoS. The capacity of sickled cells to unsickle during reoxygenation is represented in the parameter Recovery (percentage of EImax reached during reoxygenation). To aid in the interpretation, all data points were connected in every individual experiment by a line to graphically present the results. This figure has been modified from Rab et al.22 Please click here to view a larger version of this figure.
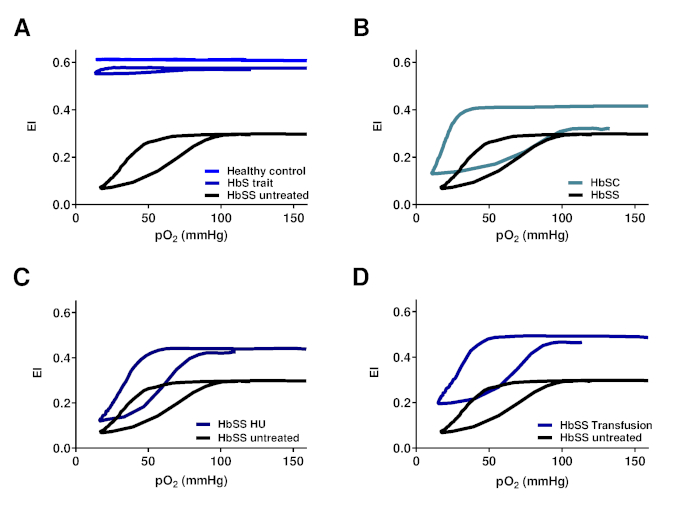
Figure 4. Oxygen gradient ektacytometry parameters correlate with genotype and treatment regimens of SCD patients with SCD. (A) Representative graph of RBCs of HbS carriers (HbS trait) and healthy controls in relation to untreated HbSS patients. (B) Representative graph of RBCs of patients with Hemoglobin SC Disease (HbSC) in relation to untreated HbSS patients. (C) Representative graph of RBCs of hydroxyurea treated homozygous SCD patients (HbSS HU) in relation to untreated HbSS patients. (D) Representative graph of RBCs of HbSS patients treated with blood transfusion (HbSS transfusion) in relation to untreated HbSS patients. This figure has been modified from Rab et al.22 Please click here to view a larger version of this figure.
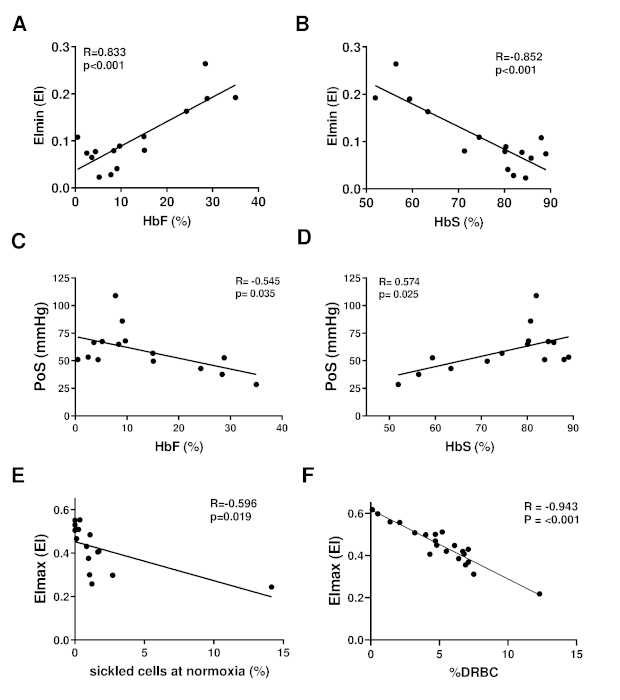
Figure 5. Oxygen gradient ektacytometry parameters are associated with %HbF, %HbS, %sickled cells at normoxia and %dense RBCs. (A) Linear correlation of minimum elongation index (EImin) and %HbF of 15 HbSS or HbS/β-thalassemia patients without transfusion. (B) Linear correlation of EImin and %HbS. (C) Linear correlation of PoS and %HbF. (D) Linear correlation of PoS and %HbS. (E) Linear correlation of maximum EI (EImax) and percent of sickled cells at normoxia measured with digital microscopy. (F) Linear correlation of EImax and percentage dense RBCs (%DRBCs) of 21 patients with HbSS. This figure has been modified from Rab et al.22 Please click here to view a larger version of this figure.
| Settings | ||
| Files | Storage directory | |
| General options | Default medium viscosity | Viscosity of PVP |
| pO2 scan | Minimum aspiration time (s) | 60 |
| pO2 scan shear stress (Pa) | 30 | |
| Determine pO2 every (S) | 20 | |
| Moving average size | 2 | |
| pO2 scan step; Edit | 0 -OFF; 60 -ON; 1360 –OFF; 1640 -OFF | |
| Cal. Area between (mmHg) | 10 and 100 | |
| pO2 control | Off (unchecked) |
Table 1. The preferred setting of the ektacytometer.
Discussion
Here we describe oxygen gradient ektacytometry, a method that can be used to study the sickling behavior of red blood cells from SCD patients under a range of oxygen concentrations (Figure 4 and Figure 5). In order to obtain reproducible results, it is important to identify the factors that influence the results. For instance, temperature has a large impact on RBC deformability, mostly due to its effects on the thickness of the viscous solution (PVP). We recommend performing a test measurement at the start of the day to thoroughly heat the machine to 37 ˚C. This will improve the reproducibility of the results. The osmolarity of the viscous solution should be within a narrow range (282–286 mOsm/kg for PVP), because osmolarity influences hydration status, which in turn affects RBC deformability. The pH and viscosity of PVP should also be tightly regulated. Differences in pH and temperature can influence curves dramatically22. Additionally, remaining water in the cup, bob, and tubes, may cause the lysis of RBCs, thereby resulting in incorrect data, because fewer intact RBCs present in the cup will be measured.
Settings to perform oxygen gradient ektacytometry can be adjusted to address specific investigational questions. Preferred settings are listed in Table 1. A deoxygenation time of 1,300 s was chosen based on observations showing that the extension of deoxygenation did not result in a lower EImin for most patients. In contrast, shortening of the deoxygenation time would hamper the discriminative power of the oxygen gradient ektacytometry. The reoxygenation time was set to 280 s due to the rapidly resolving HbS polymers during reoxygenation, and concomitant restoration of EI towards values measured prior to the deoxygenation. Shear stress was set to 30 Pa, which is analogous to the osmotic gradient ektacytometry. Lowering this parameter could hamper the discriminative power. Deoxygenation control can be used if a set of deoxygenation speed is applied to every patient sample. In our preferred settings, this option was switched off because the rate of deoxygenation is patient-specific due to the unique hemoglobin dissociation curve. Hence, switching on the deoxygenation control would eliminate this characteristic from the assay. However, this feature of oxygen gradient ektacytometry is still under investigation.
Several well-known factors influence oxygen gradient ektacytometry parameters, namely pH, temperature, and osmolarity. Ektacytometry, especially PoS, is influenced by 2,3-diphosphoglycerate (2,3-DPG)22. Also, there is a clear correlation between %HbF and the EImin, and to a lesser extent PoS (Figure 5A–D). EImax is associated with sickle cells at normoxia, which can explain the observation that shortly after a VOC, RBC deformability at normoxia (EImax), is higher. The latter is caused by the destruction of the most sickled cells, and hence less deformable RBCs during VOC16. As shown in Figure 5F, higher %dense RBCs (defined as RBCs with a hemoglobin concentration >1.11 mg/mL) correlate strongly with a lower EImax. This indicates that dense cells are an important factor in RBC deformability at normoxia, similar to previously reported results1.
Standardization of samples is very important for obtaining reproducible results and for distinguishing between different genotypes and treatments. Correcting for RBC count is important, as the number of RBCs influence the intensity of the diffraction pattern. If lower RBC numbers are present in the gap between the bob and cup, the curve will shift upward and to the left. Additionally, the curve will fluctuate, hampering accurate calculation of the parameters, especially the PoS.
A limitation of this technique is that the EI value represents an average of all cells, including different subpopulations. Heterogeneity of RBC populations in SCD patients and its influence on ektacytometry measurement has been intensively studied. This resulted in standardization wherein the size of the diffraction pattern is adjusted to a fixed value instead of corrected for the RBC count23,24. Whether or not this way of standardization should also be applied to oxygen gradient ektacytometry measurements is currently under study.
Several techniques to measure RBC deformability under hypoxic conditions were developed based on a deoxygenation step that took place outside the ektacytometer25,26,27. Under these conditions, differences in cellular behavior were not observed between patients with HbS traits and healthy controls under physiological pH25. Oxygen gradient ektacytometry, however, clearly shows a low but evident PoS in individuals with HbS traits (Figure 4A). To date, in routine clinical practice, the only alternative methods to measure the tendency of an individual patient’s RBCs to sickle in vitro include a morphology-based sickling assay: RBCs are incubated under conditions that promote HbS polymerization, such as low oxygen tension or low pH. A fixative is added after incubation and the percentage of sickled cells is manually or digitally counted using light microscopy. Many preclinical and early phase pharmacologic trials use the sickling assay to generate a secondary outcome variable to be able to predict clinical efficacy in SCD28,29,30,31,32. However, it is time consuming, variability is high and sensitivity is low, the technique is not automated and, therefore, labor intensive. Moreover, morphological changes due to sickling might not correlate well with physiological parameters, such as RBC deformability, because it is a 2-dimensional static assay2.
Oxygen gradient ektacytometry provides a functional assay of sickling that is rapid and reproducible. This is an in vitro test that does not consider the endothelial surface. However, it does provide functional aspects of sickling behavior and RBC characteristics, making it a promising technique for sickle cell studies. Future applications of the technique include monitoring treatment efficacy in SCD patients, serving as a biomarker for new treatment strategies, studying sickling behavior, and monitoring chimerism after the stem cell transplantation in SCD.
Disclosures
The authors declare no competing financial interests.
Acknowledgements
This work was supported in part by a Eurostars grant estar18105 and by an unrestricted grant provided by RR Mechatronics. The authors thank Sisto Hendriks and Jan de Zoeten for their technical support.
Materials
| Name | Company | Catalog Number | Comments |
| ADVIA 120 Hematology Analyzer | Siemens | 067-A004-14 | Instrument |
| Cell-Dyn Sapphire Hematology Analyzer | Abbott | 8H00-01 | Instrument |
| Lorrca | RR Mechatronics | LORC109230 or LORC109110 | Instrument |
| Lorrca Software version V5.08 | RR Mechatronics | - | Software |
| Nitrogen gas 4.8 or 5.0 | Local | - | |
| O2-spot | RR Mechatronics | PO2S020153 | O2 measurement |
| Oxygenscan module (pO2scan) | RR Mechatronics | PO2S109000 | Add-on |
| Oxy-ISO | RR Mechatronics | QRR 030905 | Viscous solution |
| X-Clean | RR Mechatronics | QRR 010946 | Cleaning solution Lorrca |
References
- Clark, M. R., Mohandas, N., Shohet, S. B. Deformability of oxygenated irreversibly sickled cells. Journal of Clinical Investigation. 65 (1), 189-196 (1980).
- Smith, C., Kuettner, J., Tukey, D., White, J. Variable Deformability of Irreversibly Sickled Erythrocytes. Blood. 58 (1), 71-78 (1981).
- Clark, M., Mohandas, N., Embury, S., Lubin, B. A simple laboratory alternative to irreversibly sickled (ISC) counts. Blood. 60 (3), 659-663 (1982).
- DaCosta, L., et al. Diagnostic tool for red blood cell membrane disorders Assessment of a new generation ektacytometer. Blood Cells, Molecules, and Diseases. 56 (1), 9-22 (2016).
- Rabai, M., et al. Deformability analysis of sickle blood using ektacytometry. Biorheology. 51 (2-3), 159-170 (2014).
- Ballas, S. K., Mohandas, N. Sickle red cell microrheology and sickle blood rheology. Microcirculation. 11 (2), 209-225 (2004).
- Connes, P., Alexy, T., Detterich, J., Romana, M., Hardy-Dessources, M. D., Ballas, S. K. The role of blood rheology in sickle cell disease. Blood Reviews. 30 (2), 111-118 (2015).
- Hierso, R., et al. Effects of oxidative stress on red blood cell rheology in sickle cell patients. British Journal of Haematology. 166 (4), 601-606 (2014).
- Mozar, A., et al. Red blood cell nitric oxide synthase modulates red blood cell deformabilityin sickle cell anemia. Clinical Hemorheology and Microcirculation. 64 (1), 47-53 (2016).
- Clark, M. R., Mohandas, N., Shohet, S. B. Osmotic Gradient Ektacytometry: Comprehensive Characterization of Red Cell Volume and Surface Maintenance. Blood. 61 (5), 899-911 (1983).
- Parrow, N. L., et al. Measurements of red cell deformability and hydration reflect HbF and HbA2in blood from patients with sickle cell anemia. Blood Cells, Molecules, and Diseases. 65, 41-50 (2017).
- Steinberg, M. H., Chui, D. H. K., Dover, G. J., Sebastiani, P., Alsultan, A. Fetal hemoglobin in sickle cell anemia: A glass half full. Blood. 123 (4), 481-485 (2014).
- Ballas, S. K., Larner, J., Smith, E. D., Surrey, S., Schwartz, E., Rappaport, E. F. Rheologic predictors of the severity of the painful sickle cell crisis. Blood. 72 (4), 1216-1223 (1988).
- Lande, W. M., et al. The Incidence of Painful Crisis in Homozygous Sickle Cell Disease: Correlation with Red Cell Deformability. Blood. 72 (6), 2056-2059 (1988).
- Lemonne, N., et al. Does increased red blood cell deformability raise the risk for osteonecrosis in sickle cell anemia. Blood. 121 (15), 3054-3057 (2013).
- Ballas, S. K., Smith, E. D. Red blood cell changes during the evolution of the sickle cell painful crisis. Blood. 79 (8), 2154-2163 (1992).
- Telen, M. Cellular adhesion and the endothelium: E-selectin, L-selectin, and pan-selectin inhibitors. Hematology/Oncology Clinics of North America. 28 (2), 341-354 (2014).
- Papageorgiou, D. P., et al. Simultaneous polymerization and adhesion under hypoxia in sickle cell disease. Proceedings of the National Academy of Sciences. 115 (38), 201807405 (2018).
- Liu, J., Qiang, Y., Alvarez, O., Du, E. Electrical impedance microflow cytometry with oxygen control for detection of sickle cells. Sensors and Actuators, B: Chemical. 255, 2392-2398 (2018).
- Du, E., Diez-Silva, M., Kato, G. J., Dao, M., Suresh, S. Kinetics of sickle cell biorheology and implications for painful vasoocclusive crisis. Proceedings of the National Academy of Sciences. 112 (5), 1422-1427 (2015).
- Li, Q., et al. Kinetic assay shows that increasing red cell volume could be a treatment for sickle cell disease. Proceedings of the National Academy of Sciences. 114 (5), 689-696 (2017).
- Rab, M. A. E., et al. Rapid and reproducible characterization of sickling during automated deoxygenation in sickle cell disease patients. American Journal of Hematology. 94, 575-584 (2019).
- Renoux, C., et al. Importance of methodological standardization of ektacytometric measures of red blood cell deformability in sickle cell anemia. Clinical Hemorheology and Microcirculation. 62 (2), 173-179 (2016).
- Parrow, N. L., et al. Measuring Deformability and Red Cell Heterogeneity in Blood by Ektacytometry. Journal of Visualized Experiments. (131), (2018).
- Bessis, M., Feo, C., Jones, E. Quantitation of red cell deformability during progressive deoxygenation and oxygenation in sickling disorders (the use of an automated Ektacytometer). Blood Cells. 8 (1), 17-28 (1982).
- Sorette, M. P., Lavenant, M. G., Clark, M. R. Ektacytometric measurement of sickle cell deformability as a continuous function of oxygen tension. Blood. 67 (6), 1600-1606 (1987).
- Huang, Z., Hearne, L., Irby, C. E., King, S. B., Ballas, S. K., Kim-Shapiro, D. B. Kinetics of increased deformability of deoxygenated sickle cells upon oxygenation. Biophysical journal. 85 (4), 2374-2383 (2003).
- Antoniani, C., et al. Induction of fetal hemoglobin synthesis by CRISPR/Cas9-mediated editing of the human β-globin locus. Blood. 131 (17), 1960-1973 (2018).
- Abdulmalik, O., et al. Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin. Acta Crystallographica Section D: Biological Crystallography. 67 (12), 1076 (2011).
- Oder, E., Safo, M. K., Abdulmalik, O., Kato, G. J., Discovery, D. New Developments in Anti-Sickling Agents: Can Drugs Directly Prevent the Polymerization of Sickle Haemoglobin In Vivo. British Journal of Haematology. 175 (1), 24-30 (2016).
- Oksenberg, D., et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. British Journal of Haematology. 175 (1), 141-153 (2016).
- Xu, G. G., et al. Synthesis, and Biological Evaluation of Ester and Ether Derivatives of Antisickling Agent 5-HMF for the Treatment of Sickle Cell Disease. Molecular Pharmaceutics. 14 (10), 3499-3511 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved