Method Article
Versatile CO2 Transformations into Complex Products: A One-pot Two-step Strategy
In This Article
Summary
CO2 transformations are conducted in a one-pot two-step procedure for the synthesis of complex molecules. The selective 4 e- reduction of CO2 with a hydroborane reductant affords a reactive and versatile bis(boryl)acetal intermediate which is subsequently involved in condensation reaction or carbene-mediated C-C coupling generation.
Abstract
CO2 transformations using a one-pot two-step method are presented herein. The purpose of the method is to give access to a variety of value-added products and notably to generate chiral carbon centers. The crucial first step consists in the selective double hydroboration of CO2 catalyzed by an iron hydride complex. The product obtained with this 4 e- reduction is a rare bis(boryl)acetal, compound 1, which is subjected in situ to three different reactions in a second step. The first reaction concerns a condensation reaction with (diisopropyl)phenylamine affording the corresponding imine 2. In the second and third reaction, intermediate 1 reacts with triazol-5-ylidene (Enders' carbene) to afford compounds 3 or 4, depending on the reaction conditions. In both compounds, C-C bonds are formed, and chiral centers are generated from CO2 as the only source of carbon. Compound 4 exhibits two chiral centers obtained in a diastereoselective manner in a formose-type mechanism. We proved that the remaining boryl fragment plays a key role in this unprecedented stereocontrol. The interest of the method stands on the reactive and versatile nature of 1, giving rise to various complex molecules from a single intermediate. The complexity of a two-step method is compensated by the overall short reaction time (2 h for the larger reaction time), and mild reaction conditions (25 °C to 80 °C and 1 to 3 atm of CO2).
Introduction
In light of the large interest in using CO2 as a sustainable carbon source1,2,3, the purpose of the method is to transform CO2 into a variety of value-added products.
Intense researches aim at functionalizing CO24,5 or reducing it into formic acid (2 e- reduction), carbon monoxide (2 e- reduction), methanol (6 e- reduction) or methane (8 e- reduction)1,6. The interception of the 2 e- reduction product with amine, notably, gives rise to formamide and methylamine7,8,9. These areas of research are the most advanced so far. However, the scope of accessible functions and the added value of products formed compared to the starting materials remains rather minimal.
To circumvent this limitation, we focused i) on the 4 e- reduction of CO2 and ii) on applying a one-pot two-step procedure. The interest of the one-pot two-step procedure is to limit compatibility issues between the two steps and consequently to broaden the type of reactivity which could be conducted after the first step of reduction. We targeted the 4 e- reduction of CO2 because formaldehyde -the simplest 4 e- reduction product- is a particularly reactive and versatile carbon source10,11. It is used in condensation reaction as a methylene source and can be polymerized into carbohydrates. The latter -called formose reaction- is an impressive transformation generating carbon chain and chiral carbon centers solely from formaldehyde and is of high interest for synthetic-12,13 and prebiotic-chemistry14,15,16.While we are able to observe free formaldehyde from CO2 hydroboration17, its selective generation under homogeneous conditions is still unprecedented. Instead of formaldehyde, we developed the synthesis of bis(boryl)acetal compound 1 from the selective double hydroboration of CO218,19.
In one-pot two step processes we prove herein that, in the same pot, this intermediate 1 i) reacts as a surrogate of formaldehyde in condensation reactions18 or ii) gives rise to a modified formose-type reaction20. In this latter reaction, C-C coupling and chiral carbon centers are obtained.
Protocol
CAUTION: Please consult material safety data sheets (MSDS) of the chemicals before use. Please use appropriate safety practices when performing the following reactions and personal protective equipment. A special attention must be dedicated to the use of the vacuum line and gas pressure system.
1. Synthesis of compound 2 from CO2 in a nuclear magnetic resonance (NMR) tube
- Stock solution of Fe(H)2(dmpe)2: dissolve 4.6 mg of Fe(H)2(dmpe)221,22 in 1 mL of tetrahydrofuran (THF)-d8.
NOTE: dmpe = 1,2-bis(dimethylphosphino)ethane - In a glove box, charge an NMR tube with 15.9 mg of 9-borabicyclo[3.3.1]nonane (9-BBN) and 100 µL of a stock solution of Fe(H)2(dmpe)2 (1 mol%).
- Add 0.5 mL of tetrahydrofuran (THF)-d8.
- Close the tube and bring it outside the glove box.
- Connect the tube to a gas system and place it at 25 °C for 15 min to equilibrate the temperature of the solution inside the tube.
NOTE: The gas system connects the CO2 bottle to both the vacuum line and the NMR tube. The connections are with Teflon tube and Swadgelock connectors (see Figure 1 for a scheme of the setup). This system enables to add the pressure defined at the regulator at the desired temperature. - Add 1 atm of CO2.
- Leave for 3 min under a dynamic pressure of CO2 and close the tube.
- Leave the tube at 25 °C for 45 min.
NOTE: At this step bis(boryl)acetal 1 is generated inside the NMR tube in 85% yield (see representative results for the NMR analysis). - Stock solution of amine: dissolve 177.3 mg of 2,6-(diisopropyl)phenylamine in 1 mL of THF-d8.Once compound 1 is generated, open the NMR tube inside a glove box and add 55 µL of a stock solution of the 2,6-(diisopropyl)phenylamine, corresponding to 1 equivalent of the generated bis(boryl)acetal 1.
- Close the tube and hand shake it for 10 s.
- After 20 min, confirm the formation of imine 2 by 1H NMR analysis (Figure 2). Use hexamethylbenzene (roughly 10 mol% vs 9-BBN) as an internal standard to determine the NMR yield.
2. Synthesis of compound 3 from CO2 in a Fisher Porter
- Charge a Fisher Porter with 320 mg of 9-BBN, 9.4 mg of Fe(H)2(dmpe)2 and a magnetic stirring bar21,22.
- Add 10 mL of THF.
- Close the Fisher Porter and bring it outside the glove box.
- Place it at 25 °C for 15 min to equilibrate the temperature of the solution.
- Connect the Fisher Porter to the gas system and add 1 atm of CO2.
NOTE: The gas system connects the CO2 bottle to both the vacuum line and the Fisher Porter. The connections are with Teflon tube and Swadgelock connectors (see Figure 1 for a scheme of the setup). This system enables to add the pressure defined at the regulator at the desired temperature. - Leave for 3 min under a dynamic pressure of CO2, close the tube and stir it at 25 °C for 45 min.
NOTE: This stage corresponds to the selective generation of bis(boryl)acetal 1 from CO2 hydroboration in 85% yield. - After 45 min, open the Fisher Porter in a glove box and add a solution of 380 mg of triazol-5-ylidene in 6 mL of THF.
- Outside the glove box, charge the Fisher Porter with 3 atm of CO2.
- Stir the solution at 60 °C for 60 min under a dynamic pressure of 3 atm of CO2.
- Let the solution cool down to room temperature.
- Remove the volatiles under vacuum and wash the residue with 3x 2 mL of diethylether (Et2O) at 0 °C to obtain CO2 adduct 3 as a white powder (Figure 3).
- To generate monocrystals, place a concentrated THF/pentane solution at -37 °C for 24-48 h.
3. Synthesis of compound 4 from CO2 in a Fisher Porter
- In a glove box, charge a Fisher Porter tube with 159 mg of 9-BBN, 4.7 mg (1 mol%) of Fe(H)2(dmpe)2 and a magnetic stirring bar.
- Add 5 mL of THF.
- Close the Fisher Porter and bring it outside the glove box.
- Place it at 25 °C for 15 min to equilibrate the temperature of the solution.
- Connect the Fisher Porter to the gas system and add 1 atm of CO2.
- Leave 3 min under a dynamic pressure of CO2, close the Fisher Porter and stir it at 25 °C for 45 min.
NOTE: This stage corresponds to the selective generation of bis(boryl)acetal 1 from CO2 hydroboration in 85% yield. - After the generation of 1, open the Fisher Porter in a glove box and add 54 mg of triazol-5-ylidene.
- Outside the glove box, stir the solution at 80 °C for 40 min to generate a mixture of compounds containing compound 4.
- Remove the solvent and dissolve part of the residue in 0.6 mL of THF-d8.
- Filtrate the solution with a syringe equipped with a PTFE filter (0.2 µm) and place it in an NMR tube for analysis. Use hexamethylbenzene (roughly 10 mol% vs 9-BBN) as an internal standard to determine the NMR yields.
4. Alternative synthesis of compound 4 from d,l-Glyceraldehyde
- In a glove box, charge a Schlenk tube with 50 mg of d,l-Glyceraldehyde, 135 mg of 9-BBN and a magnetic stirring bar.
- Add 4 mL of THF.
CAUTION: Upon dissolution of the compound, H2 evolution occurs. - Close the Schlenk with a septum and stick a needle into it to allow the constant release of H2 formed.
- Stir the white suspension at room temperature for 24 h inside the glove box.
- Add 165 mg of triazol-5-ylidene.
- Stir 3 h at room temperature. All residues solubilize during that time.
- Remove the volatiles under vacuum.
- Solubilize the residue in a minimum amount of Et2O (1 mL) and place the solution at -37 °C for 12 h.
- Compound 4 precipitates. Remove the filtrate by filtration and dry the precipitate under vacuum.
- Isolate compound 4 as a white powder in 72% yield.
Results
Successful generation of bis(boryl)acetal compound 1 is assessed by 1H NMR analysis with the characteristic methylene pick at 5.54 ppm in THF-d8 (Figure 4a). Successful generation of compound 2 is assessed by 1H NMR analysis with the characteristic AB signal (δ = 7.73 (d, 1H, 2JH-H = 18.4 Hz, CH2), 7.30 (d, 1H, 2JH-H = 18.4 Hz, CH2) for the two inequivalent protons of the methylene in THF-d8 (Figure 4b). Successful generation of compound 3 is assessed by 1H NMR analysis in THF-d8 (Figure 4c). The most notable signals are the CHCO2 at 5.34 ppm, and the CH of the BBN fragment at 0.26 and -0.65 ppm. Successful generation of compound 4 is assessed by 1H NMR analysis in THF-d8. As shown in Figure 4d, compound 4, in situ generated from CO2, is notably characterized by a doublet at 4.64 ppm (3JH-H = 7.9 Hz, H3) and a pseudo-t at 3.36 (2JH-H = 9.7 Hz, 3JH-H = 9.5 Hz, 1H, H1b). In the isolated compound 4 from d,l-Glyceraldehyde, the four proton signals of the C3 chain are clearly observed (Figure 4e) and the three carbon atoms of the chain are characterized in the 13C{1H} NMR analysis at 76.9 (C2), 74.0(C3) and 71.5(C1) ppm (Figure 5).
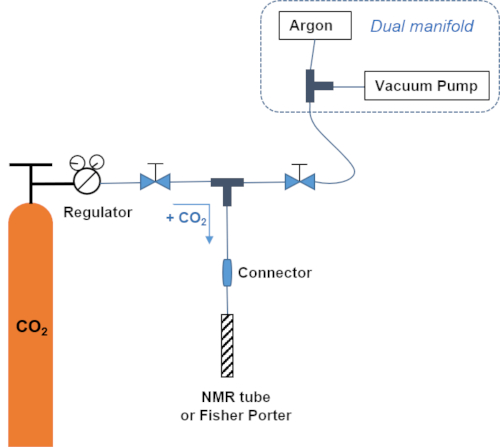
Figure 1: Gas system. Scheme of the gas system enabling the addition of a defined pressure of CO2 at a given temperature. Please click here to view a larger version of this figure.
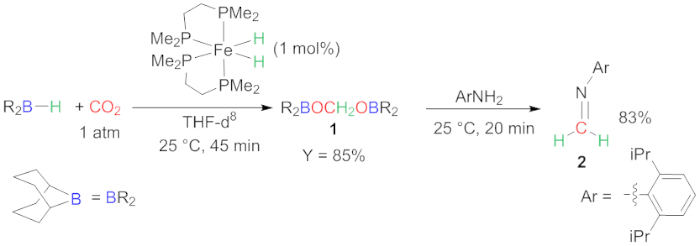
Figure 2: Reductive functionalization of CO2. Synthesis of compounds 1 and 2. Please click here to view a larger version of this figure.
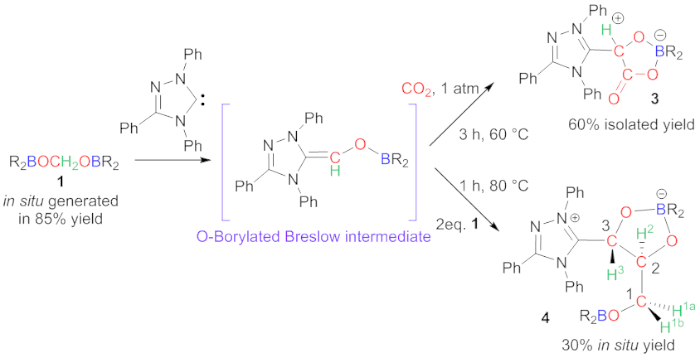
Figure 3: Carbene-mediated C-C bond formation. Synthesis of compounds 3 and 4. Please click here to view a larger version of this figure.
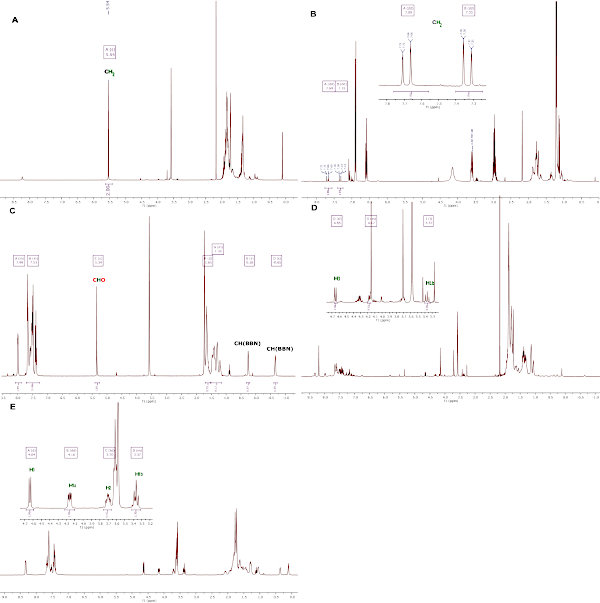
Figure 4: 1H NMR analyses of compound 1-4, recorded at room temperature in THF-d8. (A) In situ generated compound 1, (B) in situ generated compound 2, (C) isolated compound 3, (D) in situ generated compound 4 from CO2, (E) isolated compound 4 from d,l-Glyceraldehyde. Please click here to view a larger version of this figure.
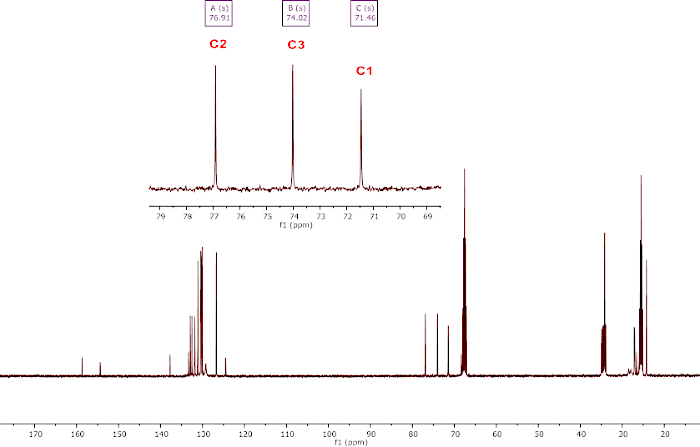
Figure 5: Representative characterization of compound 4 isolated from d,l-Glyceraldehyde. 13C{1H} NMR analysis recorded at room temperature in THF-d8; inlet: zoom of the C1-C3 area. Please click here to view a larger version of this figure.
Discussion
Herein, we present the one-pot two-step versatile transformations of CO2 into complex products. The first step of the method concerns the selective 4 e- reduction of CO2 with a hydroborane reductant. This step is critical because selectivity toward the 4 e- reduction is challenging. Very few systems have been reported that describe the selective generation of bis(boryl)acetal23,24,25. In our case, an iron hydride complex catalyzes this selective 4 e- reduction of CO2 with 9-BBN, affording compound 1, under mild conditions (25 °C) and with very short reaction time (45 min) (Figure 2)18. Our study shows that the reaction conditions are very important. In our hand, each attempt to change the concentration, solvent, CO2 pressure and temperature led to the decrease of the yield in compound 1. A longer reaction time is also detrimental because it leads to over-reduction to the methanol level or evolution of the bis(boryl)acetal into several oligomeric compounds. From our experience, it is necessary to verify the outcome of this reduction step by in situ 1H NMR characterization. The reproducibility of the method needs to be probed over several runs.
The in situ condensation reaction of the intermediate 1 with a bulky aniline gives rise to the corresponding imine 2 (Figure 2). This is a straightforward method and compound 2 is readily formed in a high yield (83%). This reaction can also be used to probe the efficiency of the reduction step. This method is the only method enabling the synthesis of imine function from CO2. Moreover, intermediate 1 was proved to be a versatile source of methylene in various condensation reactions leading to the formation of C-N, C-O, C-C and C=C bonds18. This method thus offers a straightforward way of using CO2 as a surrogate of formaldehyde in condensation reactions26.
Intermediate 1 reacts with Ender's carbene to afford compounds 3 or 4, depending on the reaction conditions (Figure 3)20. With the support of in-depth experimental and theoretical study, we were able to explain the observed reactivity. In this case, compound 1 does not react as formaldehyde since boryl moieties remain in compounds 3 and 4. This feature arises from the formation of an unprecedented O-Borylated Breslow intermediate (Figure 3)27,28,29,30,31,32. This intermediate is not observed experimentally but may act as a bifunctional Lewis acid/Lewis base activator toward CO2 to afford compound 3 or leads to the homocoupling of two more carbon centers to afford compound 4. In both products, chiral centers are generated and in the case of compound 4, the two chiral centers, C2 and C3, are obtained in a diastereoselective manner, thanks to the presence of the bridging boryl fragment.
The advances presented herein were possible thanks to the one-pot two-step method employed and to the high and versatile reactivity of intermediate 1 generated from the selective 4e- reduction of CO2. Following a similar method to further improve the scope and the complexity of the synthesized molecules, on-going works are dedicated i) to tune the properties of bis(boryl)acetal in using other hydroborane reductants and ii) to probe different coupling conditions in using other organo-catalysts.
Disclosures
The authors have nothing to disclose.
Acknowledgements
S. D. thanks Région Midi-Pyrénées and Université Fédérale de Toulouse for doctoral fellowship. D. Z. thanks Chinese Scholarship Council for doctoral fellowship. A. M. thanks COLFUTURO for doctoral fellowship. S. B. thanks the ANR programme JCJC "ICC" and Prof. A. Leon for fruitful discussion.
Materials
| Name | Company | Catalog Number | Comments |
| Wilmad quick pressure valve NMR tube 5 mm diam. | Sigma-Aldrich | Z562882-1EA | |
| Filtre-seringue PTFE hydrophobe Ø 25 mm pores 0,22 µm | UGAP | 2528593 | |
| Fisher Porter | Home made system | ||
| 9-borabicyclo[3.3.1]nonane dimer | Sigma-Aldrich | 178713 | |
| FeCl2 (anhydrous) | Strem | MFCD00011004 | |
| d,l-Glyceraldehyde | Sigma-Aldrich | G5001 | |
| 2,6-(diisopropyl)phenylamine | Sigma-Aldrich | 374733 | |
| dimethylphosphinoethane | Strem | MFCD00008511 | |
| Tetrahydrofuran | Carlo Erba | solvent | |
| Diethyl ether | Carlo Erba | solvent | |
| Pentane | Carlo Erba | solvent | |
| Tetrahydrofuran D8 | Eurisotop | D149FE |
References
- Goeppert, A., Czaun, M., Jones, J. P., Surya Prakash, G. K., Olah, G. A. Recycling of carbon dioxide to methanol and derived products - closing the loop. Chemical Society Reviews. 43, 7995-8048 (2014).
- Appel, A. M., et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chemical Reviews. 113 (8), 6621-6658 (2013).
- Aresta, M., Dibenedetto, A., Angelini, A. Catalysis for the Valorization of Exhaust Carbon: from CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chemical Reviews. 114 (3), 1709-1742 (2014).
- Poland, S. J., Darensbourg, D. J. A quest for polycarbonates provided via sustainable epoxide/CO2 copolymerization processes. Green Chemistry. 19, 4990-5011 (2017).
- Kember, M. R., Buchard, A., Williams, C. K. Catalysts for CO2/epoxide copolymerisation. Chemical Communications. 47 (1), 141-163 (2011).
- Klankermayer, J., Wesselbaum, S., Beydoun, K., Leitner, W. Selective Catalytic Synthesis Using the Combination of Carbon Dioxide and Hydrogen: Catalytic Chess at the Interface of Energy and Chemistry. Angewandte Chemie International Edition. 55 (26), 7296-7343 (2016).
- Tlili, A., Blondiaux, E., Frogneux, X., Cantat, T. Reductive functionalization of CO2 with amines: an entry to formamide, formamidine and methylamine derivatives. Green Chemistry. 17, 157-168 (2015).
- Bontemps, S. Boron-mediated activation of carbon dioxide. Coordination Chemistry Reviews. 308 (Part 2), 117-130 (2016).
- Chong, C. C., Kinjo, R. Catalytic Hydroboration of Carbonyl Derivatives, Imines, and Carbon Dioxide. ACS Catalysis. 5 (6), 3238-3259 (2015).
- Reuss, G., Disteldorf, W., Gamer, A. O., Hilt, A. Formaldehyde. Ullmann's Encyclopedia of Industrial Chemistry. , Wiley, Weinheim. (2003).
- Heim, L. E., Konnerth, H., Prechtl, M. H. G. Future perspectives for formaldehyde: pathways for reductive synthesis and energy. Green Chemistry. 19, 2347-2355 (2017).
- Zafar, I., Senad, N. The Formose Reaction: A Tool to Produce Synthetic Carbohydrates Within a Regenerative Life Support System. Current Organic Chemistry. 16 (6), 769-788 (2012).
- Delidovich, I. V., Simonov, A. N., Taran, O. P., Parmon, V. N. Catalytic Formation of Monosaccharides: From the Formose Reaction towards Selective Synthesis. ChemSusChem. 7 (7), 1833-1846 (2014).
- Ruiz-Mirazo, K., Briones, C., de la Escosura, A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chemical Reviews. 114 (1), 285-366 (2014).
- Ricardo, A., Carrigan, M. A., Olcott, A. N., Benner, S. A. Borate Minerals Stabilize Ribose. Science. 303 (5655), 196(2004).
- Hein, J. E., Blackmond, D. G. On the Origin of Single Chirality of Amino Acids and Sugars in Biogenesis. Accounts of Chemical Research. 45 (12), 2045-2054 (2012).
- Bontemps, S., Vendier, L., Sabo-Etienne, S. Ruthenium-Catalyzed Reduction of Carbon Dioxide to Formaldehyde. Journal of the American Chemical Society. 136 (11), 4419-4425 (2014).
- Jin, G., Werncke, C. G., Escudié, Y., Sabo-Etienne, S., Bontemps, S. Iron-Catalyzed Reduction of CO2 into Methylene: Formation of C-N, C-O, and C-C Bonds. Journal of the American Chemical Society. 137 (30), 9563-9566 (2015).
- Bontemps, S., Vendier, L., Sabo-Etienne, S. Borane-Mediated Carbon Dioxide Reduction at Ruthenium: Formation of C1 and C2 Compounds. Angewandte Chemie International Edition. 51 (7), 1671-1674 (2012).
- Béthegnies, A., et al. Reductive CO2 Homocoupling: Synthesis of a Borylated C3 Carbohydrate. ChemCatChem. 11 (2), 760-765 (2019).
- Dombray, T., et al. Iron-Catalyzed C-H Borylation of Arenes. Journal of the American Chemical Society. 137 (12), 4062-4065 (2015).
- Allen, O. R., et al. Addition of CO2 to Alkyl Iron Complexes, Fe(PP)2Me2. Organometallics. 27 (9), 2092-2098 (2008).
- Das Neves Gomes, C., Blondiaux, E., Thuéry, P., Cantat, T. Metal-Free Reduction of CO2 with Hydroboranes: Two Efficient Pathways at Play for the Reduction of CO2 to Methanol. Chemistry - A European Journal. 20 (23), 7098-7106 (2014).
- Murphy, L. J., et al. Selective Ni-Catalyzed Hydroboration of CO2 to the Formaldehyde Level Enabled by New PSiP Ligation. Organometallics. 36 (19), 3709-3720 (2017).
- Courtemanche, M. A., et al. Intramolecular B/N frustrated Lewis pairs and the hydrogenation of carbon dioxide. Chemical Communications. 51, 9797-9800 (2015).
- Frogneux, X., Blondiaux, E., Thuéry, P., Cantat, T. Bridging Amines with CO2: Organocatalyzed Reduction of CO2 to Aminals. ACS Catalysis. 5 (7), 3983-3987 (2015).
- Berkessel, A., Yatham, V. R., Elfert, S., Neudörfl, J. M. Characterization of the Key Intermediates of Carbene-Catalyzed Umpolung by NMR Spectroscopy and X-Ray Diffraction: Breslow Intermediates, Homoenolates, and Azolium Enolates. Angewandte Chemie International Edition. 52 (42), 11158-11162 (2013).
- DiRocco, D. A., Rovis, T. Catalytic Asymmetric Cross-Aza-Benzoin Reactions of Aliphatic Aldehydes with N-Boc-Protected Imines. Angewandte Chemie International Edition. 51 (24), 5904-5906 (2012).
- Maji, B., Mayr, H. Structures and Reactivities of O-Methylated Breslow Intermediates. Angewandte Chemie International Edition. 51 (41), 10408-10412 (2012).
- Bugaut, X., Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chemical Society Reviews. 41 (9), 3511-3522 (2012).
- Paul, M., et al. Breslow Intermediates from Aromatic N-Heterocyclic Carbenes Benzimidazolin-2-ylidenes, Thiazolin-2-ylidenes). Angewandte Chemie International Edition. 57, 8310-8315 (2018).
- Holland, M. C., Gilmour, R. Deconstructing Covalent Organocatalysis. Angewandte Chemie International Edition. 54 (13), 3862-3871 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved