Method Article
Targeting the Rat's Small Bowel: Long-Term Infusion into the Superior Mesenteric Artery
In This Article
Summary
Access for long-term infusion in the superior mesenteric artery (SMA) of rats is a surgical procedure that consists of cannulation of a proximal branch of the SMA. The cannula exits from the abdominal wound and is tunneled through the subcutaneous space back to the interscapular fold.
Abstract
The superior mesenteric artery can be cannulated in humans through minimally invasive radiological catheterization of the femoral or axillary artery. SMA cannulation is more difficult in rats due to small anatomical dimensions. The aim of the study is to describe a surgical technique for cannulation of the SMA in rats to perform long-term infusion of drugs into the SMA vascular bed in unrestricted animals, which will result in a high rate of catheter patency after the post surgical recovery for 24 hours.
To avoid the risk of SMA thrombosis or bleeding from direct access, a proximal branch of the SMA is isolated, ligated distally and cannulated with a 0.25 mm polyurethane capillary tube whose tip is advanced close to the origin of the SMA from the aorta. The cannula is then tunnelled subcutaneously to the back of the animal's neck and through the skin via an artificial valve. The external portion of the cannula is inserted in a semi-rigid support system and connected to the continuous infusion pump outside the cage where the rat is free to move.
Correct positioning of the cannula was demonstrated by post-surgical angiography and autopsy findings. Catheter patency after 24 hours of saline infusion into the SMA region was assured in most rats by the total discharge of the pump and recognition of a functional cannula for blood sampling or saline infusion.
Introduction
The superior mesenteric artery (SMA) in humans as in rats originates from the abdominal aorta and supplies the bowel with arterial blood from the duodenum to the proximal transverse colon. SMA gives rise to numerous branches.
After capillary perfusion, the mesenteric circulation is drained through the portal vein to the liver, where it undergoes hepatic metabolism before being readmitted to the systemic circulation. Cannulation of the SMA is useful for diagnostic purposes, therapeutic embolization and drug infusion in a selective or continuous manner to evaluate the effect on the bowel or, most importantly, the liver metabolism and chemical clearance. In humans, minimally invasive radiological catheterization of the SMA is performed for endovascular treatment1 or selective drug infusion2 using several percutaneous approaches like transfemoral or transaxillary puncture and cannulation.
There are literature reports of different techniques of cannulation of the small abdominal vessels: the superior mesenteric vein (SMV)3, the inferior mesenteric artery (IMA)4, the mesenteric lymph duct5, the hepatic artery6 or studies for ex vivo on bowel perfusion7 in rats. In comparison with the venous side, cannulation of the SMA in rats is much more demanding because of the simultaneous risks of thrombosis and bleeding, provided its high pressure. In particular, problems arise in case the cannulation is in operation when the rat awakes from anaesthesia on the surgical bed and more if the experiment requires a free animal in a cage after surgery.
A recent paper has described SMA cannulation as a part of the experiment (blood pressure measure) in an animal under anaesthesia8. However, no technique is described on the surgical cannulation of the SMA for long-term infusion in an unrestricted animal. The aim of this manuscript is to describe step by step a surgical technique for long term cannulation of the SMA through a proximal branch, which allows for the selective infusion of drugs into the mesenteric bed for at least 24 hours (and over). As a steady and sturdy cannulation requires permanent ligation and closure of the vessel where the catheter is inserted, this technique instead avoids inserting the catheter directly into the SMA9 and approaches the vessel through the cannulation of a proximal branch, as proximal as possible to the very origin of the SMA from aorta. Proximal infusion allows the infused drug to reach the widest anatomic bed possible, without closing the blood flow through the principal vessel.
The rat SMA cannulation technique has many applications. It would be possible to administer drugs selectively in the mesenteric arterial compartment to obtain local action at the gastrointestinal level and to avoid systemic effects and hepatic drug metabolism. The SMA-cannulated rat model has advantages over larger animal models: it is less expensive, it is ethically acceptable, and it is easier to perform and learn. SMA cannulation surgery is also easier to perform in the rat model compared to the mouse model.
Protocol
The studies described in this manuscript were approved by the local animal Ethics Committee (Università Cattolica del Sacro Cuore, Roma) and were conducted in accordance with the Italian Ministry of Health.
1. Preparation of the cannula for insertion into the proximal branch of the SMA
- Cut the larger cannula of 0.93 mm O.D, 0.5 mm I.D. to the required length (about 30 cm).
- Cut the smaller cannula (0.4 mm O.D, 0.25 mm I.D.) to about 5 cm in length and insert it 1 cm into the larger cannula.
- Fix the two cannulas together by cyanoacrylate glue, avoiding occlusion of the lumen.
- Connect the free extremity of larger cannula to a Luer stub adapter (23 G) mounted on a 1 mL syringe filled with saline solution.
- Sharpen the free tip of the smaller cannula with scissors to facilitate insertion of the catheter in the branch of the SMA.
- Check the patency of the cannula by flushing with saline solution.
NOTE: The sharp end of the cannula will not damage the artery during the animal's movement because it will be fixed and will not slide along the vessel.
2. Preparation of the rat for the surgical procedure
- Perform intramuscular anaesthesia with ketamine/xylazine (100/10 mg/kg).
NOTE: Sufficient anaesthetic depth is judged by the absence or near-absence of the paw-pinch reflex. - Shave the fur from the surgical regions: the abdomen for the branch of the SMA cannulation, and the back of the neck for the cannula exit.
- Clean the surgical regions aseptically using a surgical the paw pinch reflex.
NOTE: All preparation should be performed with aseptic technique. - scrub or solution applied in a circular motion, followed by sterile saline or 70% ethanol, 3 times.
- Place the animal in a supine position, immobilizing the four limbs.
3. Cannulation of a proximal branch of the SMA
- Ensure proper anesthetic depth by testing the paw pinch reflex prior to incision.
- Apply a sterile surgical, water-resistant drape.
- With a scalpel blade, open the abdominal wall with a straight 3 cm incision on the midline of the mesogastric region through all the abdominal planes into the peritoneum.
- Place gauzes, soaked with saline solution, around the laparotomy incision on top of the surgical drape. Use sutures to keep the surgical incision open.
NOTE: All swabs and surgical instruments must be sterile. - Use cotton swabs to identify and expose the small intestine. Follow its natural disposition to identify the mesentery. Extract the mesentery out of the laparotomic cut and lay it downward on the gauzes (Figure 1A).
- Identify the SMA by feeling the pulsation.
- Use the cotton swabs to "make way" between the mesenteric fat and uncover the SMA and 2-3 of its proximal branches.
- Choose a proximal branch of the SMA sufficiently large to allow the surgical maneuvers of cannulation. Tie this branch (with a 4-0 silk suture) 3-4 cm downstream from its origin to allow its expansion keeping the suture ends long enough to be manipulated later.
- Place a rigid support under the branch of the SMA. The handle of the surgical forceps is sufficient here.
- Hold the extremity of the smaller cannula (linked with the bigger cannula at the opposite extremity) with the dominant hand using forceps and pull the suture ends with the other hand to strain the vessel and to facilitate the entry of the catheter (Figure 1B).
- Hold the tip of the cannula at a 20° angle from the plane of the vessel in the direction opposite to the blood flow.
- Lightly press the tip to penetrate the artery wall and insert the cannula.
NOTE: Cannulation is performed without cutting the artery; the tip of the catheter will break the vessel wall and facilitate the entry. Blood flowing back into the cannula confirms the correct insertion. - Continue the insertion of the cannula for another 1 cm in the arterial branch close to the origin from the SMA.
- Fix the cannula to the artery with a surgical knot (4-0 silk) and verify its correct functioning by flushing 1 mL of sterile saline solution or with a blood sampling.
4. Tunneling of the cannula and placement in the infusion support system
- Place a sterile surgical drape on the incision prior to changing the animal's position.
NOTE: Tunnel from back to abdomen is created by exerting pressure in the subcutaneous space with a pointed surgical instrument. A sterile surgical drape on the abdomen and back incisions should be used. - Make a 1 cm incision of the posterior region of the neck and accommodate a spherical valve.
- Pass the cannula from the laparotomy access to the valve placed in the neck through subcutaneous tissues (Figure 2A). Close the cannula distal extremity with a catheter plug to avoid air inflow.
- Replace the small bowel in the abdominal cavity. Close the abdominal wall and close the skin incisions with a continuous 3-0 silk suture.
- Secure the valve to the neck skin with stitches. Close the skin incisions with a continuous 3-0 silk suture.
5. Post-operative management
- Dress the rat with a jacket to protect the button valve. Protect the exposed part of the cannula with a steel rod during infusion and secure it to the valve (Figure 2B).
NOTE: Since surgery is performed under aseptic technique, antibiotics are not indicated. NSAID should be administered pre-operatively for pain control (5 mg/mL Meloxicam injectable, 1 mg/kg once daily for up to 3 days). After operation, stabilize the rat in a metabolic cage for the time of infusion (24h). Then re-anesthetize the rat with isoflurane inhalation for the time necessary to disassemble the infusion system. Subsequently it is possible to house the rat in a normal cage with a 12-hour light/dark cycle and free access to food and water. - Stabilize the rat in a metabolic cage for the time of infusion. The rat is now awake and free to move and eat in the cage.
- Connect the distal extremity of the cannula to an elastomeric pump (100 mL volume max, 5.0 mL/h flow rate) filled with 50 mL of sterile saline solution. Proceed with infusion for 24 hours (Figure 2C).
- On the first day, administer intramuscular antibiotics (enrofloxacin 10 mg/kg for the first 24 h) and then pass to oral administration (enrofloxacin 100 mg in 500 mL in drinking water). Dispense analgesic therapy intramuscularly during awakening (ketoprofen 5.0 mg/kg) and in the following days orally (paracetamol 200 mg in drinking water).
NOTE: Dilute oral therapy administered in drinking water to obtain a bearable taste. Monitor body weight measurement and hydration. - At the end of the infusion time (24 hours) disassemble the animal's external infusion system by removing the pump, the jacket, the steel rod, and the valve from the rat. Close and cut the cannula as it comes out of the neck, leaving this extremity under the skin of the neck after wound suture.
NOTE: In this phase it may be necessary to anesthetize rats for few minutes by isofluorane inhalation. - House the rat, individually, in a normal cage with a 12-hour light/dark cycle and free access to food and water.
NOTE: Baseline post-op food intake is about 30 g./day Baseline water intake is about 50 ml/day. Average weight should be about 400 mg.
Results
In this study, the procedure was performed on 15 rats. At the end of 24 hours of saline infusion, no signs of saline or blood loss have been observed in the metabolic cages and the abdominal wound was clean in all animals as were the cages.
In the normal cages, rats were observed for 5 days with daily monitoring of weight and water/food intake. During this period, the general condition of the animals at gross examination was good with no indications of behavioral abnormalities. All rats immediately after surgery started feeding again. The average daily food and water intake increased progressively until normal after 3 days as shown in Figure 3A and 3B, respectively. In Figure 3C, it is possible to see that weight gain was regular, gradually increasing until the end of the observation period. No alterations of bowel movements took place and daily feces and urine output were normal.
After 24 hours, there was saline residue (respectively, 40 mL and 20 mL) in only 2 pumps filled with 50 mL of saline solution while all others (86.7%) were empty. Furthermore, after this infusion period, 12 cannulas (80%) were still functional for both blood sampling and saline infusion (5 mL), while 3 cannulas were not patent anymore (2 of these were the cannulas connected to the pumps with residue) (Table 1).
At necropsy, 100% of cannulas (n=15) were still located in the SMA branch and no rats had signs of bowel ischemia (Figure 4B) or intrabdominal bleeding. The 3 occluded cannulas were found kinked respectively at 0.5 cm, 1 cm and 1.5 cm from the insertion in the SMA branch. This phenomenon is probably due to the movements of the animals in the cages.
In 5 rats, immediately after the procedure and before pump connection, 2 mL of iodinated contrast medium were injected into the mesenteric cannula, to obtain an angiography through an image intensifier (angiography was performed intraoperatively). In each rat (n=5), it was possibile to see the mesenteric arterial circle and the SMA and its main branches without contrast medium spreading in the abdomen as shown in Figure 4A. This confirmed that the cannula was well placed and fixed to the branch of the SMA.

Figure 1: Experimental photographs. (A) The small intestine following its natural disposition on a gauze (it is possible to visualize the SMA with all the branches); (B) The operator inserting the cannula into the SMA branch. It is necessary to have a solid support under the vessel to guarantee the insertion of the tube. The distal silk suture closes the vessel and the proximal one fixes the catheter inside the branch. Please click here to view a larger version of this figure.

Figure 2: Infusion support system. (A) Once tunneled subcutaneously, the cannula emerges from the posterior region of the neck through the white valve; (B) A rat wearing a jacket to stabilize the white valve. A steel rod protects the catheter during infusion. (C) Diagramatic representation of a rat housed in a metabolic cage during infusion of saline with an elastomeric pump connected to the cannula exiting the steel rod. Please click here to view a larger version of this figure.

Figure 3: Representative data for food intake, water intake and weight gain of rats (n=15) in observation period of 5 days. The average daily food (A) and water (B) intake progressively increases, and it stabilizes at physiological levels after 3 days. The average weight gain (C) gradually increases until the end of the observation period. Please click here to view a larger version of this figure.
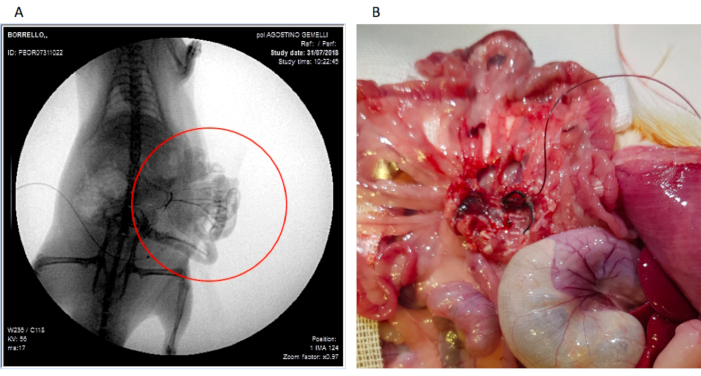
Figure 4: Photographs of (A) the contrast angiography of the mesenteric arterial region after contrast infusion through the cannula (proof of adequately placed cannula) and (B) the cannula still well positioned during autopsy. Please click here to view a larger version of this figure.
| Elastomeric pump | Cannula | |||
| Empty | With residue | Patent | Not Patent | |
| n=15 | 13 | 2 | 12 | 3 |
| % | 86.7 | 13.3 | 80 | 20 |
Table 1: Elastomeric pump dischargeand cannula patency after 24 hours of saline infusion. Patency was tested by drawing blood with a syringe and infusing 5 mL of saline in the cannula.
Discussion
The main advantage of this rat SMA infusion model is its steadfastness and durability for at least 24 hours in the vast majority of the animals. The infusion of anti-coagulant might lengthen this time interval. The model allows a reliable infusion of drugs selectively in the mesenteric region, targeting the small bowel and the proximal part of the colon.
Several steps are critical to the success of the technique. To achieve cannulation in a very small vessel it is important to select rats weighing at least 400 g; the sex and age are not relevant. It is also important to choose the correct surgical instruments and the type of cannula. Here, a smaller polyurethane cannula (0.4 mm O.D, 0.25 mm I.D.) is inserted 1 cm into the larger cannula (0.93 mm O.D, 0.5 mm I.D.) to obtain a functional and useful catheter to allow both connections to the small artery and to the larger infusion system.
The first surgical critical step is cleaning the SMA and the branch identified for cannulation from the surrounding adipose tissue (step 3.5). This helps avoid the insertion of the cannula between the tissue and the artery, which is a common mistake. However, this cleaning step is difficult as the little branch of the SMA is fragile and easy to damage. If the branch is injured, it is possible to stop the bleeding by ligature and to choose a different proximal branch, as to not to waste the animal.
To prevent air bubble formation within the cannula and avoid gas embolism, the cannula must be filled with saline until the tip before insertion in the branch. To secure the cannula in place, the application of surgical thread (4-0 silk) must be between the point of insertion into the artery and the cannula tip, directly on top of the vessel around the catheter. The surgical knot must be tight enough to fix the cannula but not too tight to occlude it (step 3.12).
The best way to ensure a correct cannulation is to see blood flow back through the cannula (step 3.10). In terms of troubleshooting, if this does not take place, it may be due to the following reasons:
the cannula was not correctly inserted into the artery;
the cannula is inside the artery but occluded by the node in an incorrect position;
the cannula is inside the artery and an air bubble in the cannula is slowing down the flow;
a clot has formed within the cannula.
An incorrect insertion may be due to cannula positioning in the space between the artery and the adipose tissue. In this case re-insertion is necessary. When the knot above the vessel occludes the cannula, it is possible to untie it very carefully and remake it. Small air bubbles in the catheter generally do not compromise the cannulation and are not life-threatening; but if there is a big air bubble in the cannula it is necessary to draw back on the cannula using the syringe or re-position the catheter in a different branch. Usually, it is possible to avoid clot formation and keep the cannula patent by infusing 0.2 mL boluses of saline once in a while during operation.
A limitation of this study is an under-evaluation of the patency of the cannula in longer infusion times: here, a 24 hour infusion was performed while rats where housed in a metabolic cage. To obtain a longer infusion period, it may be useful to use anti-coagulant therapy, not administered in this study. However, during infusion, the rat must be housed in the metabolic cage because it is the only one that supports the infusion system. This location is uncomfortable for the animal which might be stressed if treated for a longer period. Furthermore, only saline solution was used for infusion, so there are no results about specific drug administration. One limitation of the method is the impossibility to infuse in the arterial branches (if present) above that used for the catheter. For this reason it is recommended to cannulate the closest branch from the aorta.
No other rat SMA long-term infusion model for unrestrained animals is present in literature. Compared to the IMA cannulation model described many years ago4, the described technique here has a wider experimental target because it allows drug infusion in the SMA perfusion area and is not limited to the colon. Recently, for the first time, selective cannulation of a branch of the SMA was used for infusion of botulinum toxin directly in the arterial mesenteric region to study the effect on the intestinal smooth muscle10, but many other drugs could be tested in future. For example, anticoagulants can be infused to study mesenteric thrombosis, or drugs with an intestinal microbiota action11 or even drugs for inflammatory bowel diseases12. Intra-arterial infusion is useful for intestinal metabolism studies in particular, because the drug effect is evaluable before the blood goes through the portal circulation where it is subject to hepatic metabolism.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the Cen.Ri.S. (Centro di ricerche sperimentali) of Università Cattolica del Sacro Cuore in Rome for permits.
Materials
| Name | Company | Catalog Number | Comments |
| Crile-Wood Needle Holder | 2Biological Instruments | Tip Shape: Straight; Tip Width: 2 mm; Clamping Length: 14 mm; Lock: Yes; Scissors: No; Alloy / Material: Stainless Steel; Length: 15 cm; Serrated: Yes | |
| Extra Fine Graefe Forceps | 2Biological Instruments | Tip Width: 0.5 mm; Tip Dimensions: 0.5 x 0.5 mm; Alloy / Material: Stainless Steel; Length: 10 cm | |
| Luer Stub Adapter | BD Intramedic | 23 gauge for use with 427410 tubing | |
| Membrane valve | Biomed | Mod 617 | |
| Poliurethane Catheter | ENKI | external diameter: 0.4 mm, internal diameter: 0.25 mm | |
| Silastic Catheter Laboratory tubing | Healthcare industries | 508-002 | |
| Spring Scissors | 2Biological Instruments | Tip Shape: Angled; Tips: Sharp; Alloy / Material: Stainless Steel | |
| Student Surgical Scissors | 2Biological Instruments | Tip Shape: Straight; Alloy / Material: Student Stainless Steel; Serrated: No; Feature: Student Quality |
References
- Zhang, Z., Chen, X., Zhu, R. Percutaneous mechanical thrombectomy treatment of acute superior mesenteric artery embolism. European Journal of Vascular and Endovascular Surgery Short Reports. 34, 17-20 (2017).
- Wang, M. Q., et al. Transradial approach for transcatheter selective superior mesenteric artery urokinase infusion therapy in patients with acute extensive portal and superior mesenteric vein thrombosis. Cardiovascular and Interventional Radiology. 33 (1), 80-89 (2010).
- Zammit, M., Toledo-Pereyra, L. H., Malcom, S., Konde, W. N. Long-term cranial mesenteric vein cannulation in the rat. Laboratory Animal Science. 29 (3), 364-366 (1979).
- Aguiar, J. L. A., et al. Technique for long-term infusion into the inferior mesenteric artery of unrestrained rats. Laboratory Animals. 22 (2), 173-176 (1988).
- Trevaskis, N. L., Hu, L., Caliph, S. M., Han, S., Porter, C. J. The mesenteric lymph duct cannulated rat model: application to the assessment of intestinal lymphatic drug transport. Journal of Visualized Experiments. (97), e52389 (2015).
- Leivestad, O., Malt, R. A. Continuous infusion into the hepatic artery and vena cava of the rat. Surgery. 74 (3), 401-404 (1973).
- Eloy, R., et al. Ex vivo vascular perfusion of the isolated rat small bowel. Importance of the intestinal brush border enzyme-release in basal conditions. European Surgical Research. 9 (2), 96-112 (1977).
- Liu, R. N., Wei, X. J., Li, S. P., Jiang, C., Zhao, Y. Comparison of invasive dynamic blood pressure between superior mesenteric artery and common carotid artery in rats. World Journal of Emergency Medicine. 11 (2), 102-108 (2020).
- Leung, F. W., et al. Superior mesenteric artery is more important than inferior mesenteric artery in maintaining colonic mucosal perfusion and integrity in rats. Digestive Diseases and Sciences. 37 (9), 1329-1335 (1992).
- Gui, D., et al. Mesenteric artery botulinum toxin (BoNT/A1) infusion selectively blocks bowel peristalsis in rats. Journal of the American Chemical Society. 231 (4), 19-20 (2020).
- Lecomte, V., et al. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 10 (5), 0126931 (2015).
- Hajj Hussein, I. A., et al. Inflammatory bowel disease in rats: bacterial and chemical interaction. World Journal of Gastroenterology. 14 (25), 4028-4039 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved