A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Generation of a Human iPSC-Based Blood-Brain Barrier Chip
In This Article
Summary
The blood-brain barrier (BBB) is a multicellular neurovascular unit tightly regulating brain homeostasis. By combining human iPSCs and organ-on-chip technologies, we have generated a personalized BBB chip, suitable for disease modeling and CNS drug penetrability predictions. A detailed protocol is described for the generation and operation of the BBB chip.
Abstract
The blood brain barrier (BBB) is formed by neurovascular units (NVUs) that shield the central nervous system (CNS) from a range of factors found in the blood that can disrupt delicate brain function. As such, the BBB is a major obstacle to the delivery of therapeutics to the CNS. Accumulating evidence suggests that the BBB plays a key role in the onset and progression of neurological diseases. Thus, there is a tremendous need for a BBB model that can predict penetration of CNS-targeted drugs as well as elucidate the BBB's role in health and disease.
We have recently combined organ-on-chip and induced pluripotent stem cell (iPSC) technologies to generate a BBB chip fully personalized to humans. This novel platform displays cellular, molecular, and physiological properties that are suitable for the prediction of drug and molecule transport across the human BBB. Furthermore, using patient-specific BBB chips, we have generated models of neurological disease and demonstrated the potential for personalized predictive medicine applications. Provided here is a detailed protocol demonstrating how to generate iPSC-derived BBB chips, beginning with differentiation of iPSC-derived brain microvascular endothelial cells (iBMECs) and resulting in mixed neural cultures containing neural progenitors, differentiated neurons, and astrocytes. Also described is a procedure for seeding cells into the organ chip and culturing of the BBB chips under controlled laminar flow. Lastly, detailed descriptions of BBB chip analyses are provided, including paracellular permeability assays for assessing drug and molecule permeability as well as immunocytochemical methods for determining the composition of cell types within the chip.
Introduction
The BBB is a highly selective barrier that separates the CNS from the circulating blood. It protects critical brain functions from potentially disruptive substances, factors, and xenobiotics while also allowing the influx of nutrients and other metabolites required to maintain brain homeostasis1. The BBB is a multicellular NVU in which pericytes, astrocyte endfeet, and neuronal processes directly contact brain microvascular endothelial cells (BMECs). These interactions allow BMECs to form specialized barrier properties that are supported by tight and adherens junctions2,3. The formation of this barrier limits the paracellular passage of molecules, but it contains polarized transporters to actively transport molecules into the CNS or back into the blood1. Due to these unique barrier properties, the BBB constitutes a major obstacle to the delivery of biopharmaceuticals into the brain, and it is estimated that less than 5% of FDA-approved small molecules can reach the CNS4.
Animal models have been widely used to study BBB penetrance and the molecular mechanisms involved in BBB development5. While animal models faithfully represent the complex multicellular in vivo environment, differences in expression and activity of BBB transporters as well as substrate specificity across species often preclude accurate extrapolation of animal data to humans6. Thus, human-based models are critical to study the human BBB and for use in the development of drugs designed to target the CNS. This need becomes even more apparent with the increasing dominance of biological, human-specific drugs in the pharmaceutical development field. Accumulating evidence suggests that a compromised BBB is associated with a number of severe CNS disorders, including brain tumors and neurological diseases7,8,9. Human models faithfully reflecting these diseases have the potential to both 1) identify novel pathways that could be targeted for drug development and 2) predict CNS penetrance, thus reducing time and resources in preclinical studies and possibly decreasing failure rate in clinical trials.
In vitro models have been widely implemented to study interactions between BMECs and other cells of the NVU and conduct screens for prospective BBB-permeable drugs10. To recreate key aspects of the human BBB, in vitro models must display physiologically relevant properties (i.e., low paracellular permeability and physiologically relevant transendothelial electrical resistance [TEER] across the endothelial monolayer). In addition, the molecular profile of an in vitro system must include expression of representative functional transport systems. Typically, in vitro models are composed of endothelial cells that are co-cultured on a semipermeable membrane with combinations of other NVU cells to enhance BBB properties11. This approach allows simple and relatively rapid assessment of barrier functionality and molecule permeability. Such cell-based BBB models can be established with animal or human cell sources, including cells isolated from surgical excisions or immortalized BMEC lines.
Recently, protocols to differentiate human pluripotent cells into BMECs were introduced as an attractive source for in vitro human BBB models12,13. Induced pluripotent stem cell (iPSC)-derived BMECs (iBMECs) are highly scalable, demonstrate crucial morphological and functional characteristics of the human BBB, and carry the genetics of the patient. In culture, iBMECs form a monolayer that expresses tight junction markers and displays in vivo-like tight junction complexes. These cells also express BBB markers, including the BBB glucose transporter, glucose transporter 1 (GLUT1). Importantly, and unlike other alternative cell sources for human BMECs, iBMECs acquire barrier properties with values as high as those measured in vivo14, polarize along the basolateral axis, and express functional efflux pumps. Furthermore, the use of iPSCs from various subjects both 1) welcomes the opportunity to test aspects of the BBB in a personalized medicine manner and 2) provides a flexible source for generating additional cell types of the NVU. Generating these cells from an isogenic cell source to create personalized BBB chips would also aid in understanding inter individual differences in drug responses, which is a major cause for resistance or compromised response to treatment observed in clinical studies.
Use of iBMECs as monolayers in a dish or on a semi permeable transwell insert represents a powerful approach for BBB modeling. These systems tend to be robust, reproducible, and cost-effective. In addition, functional analyses such as TEER and permeability are relatively simple to perform. However, two-dimensional (2D) systems fail to recapitulate the 3D nature of in vivo tissue, and they lack the physiological shear stress forces provided by circulating blood and blood cells. This limits the ability of the vascular endothelium in these models to develop and maintain intrinsic BBB properties and functions.
Microengineered systems lined by living cells have been implemented to model various organ functionalities in a concept called organ-on-chips. By recreating in vivo-like multicellular architecture, tissue-tissue interfaces, physicochemical microenvironments, and vascular perfusion, these microengineered platforms generate levels of tissue and organ functionality not possible with conventional 2D culture systems. They also enable high resolution, real-time imaging, and analysis of biochemical, genetic, and metabolic profiles similar to living cells in the in vivo tissue and organ context. However, a particular challenge of the organ-on-chip is that the design, fabrication, and application of these microengineered chips requires specialized engineering expertise that is usually lacking in biologically oriented academic labs.
We have recently combined iPSC and organ-on-chip technologies to generate a personalized BBB chip model15,16. In order to overcome the technological challenges described, the commercially available Chip-S1 is used together with the culture module, an instrument designed to automate the maintenance of the chips in a simple and robust manner (Emulate Inc.). The BBB chip recreates interactions between neural and endothelial cells and achieves physiologically relevant TEER values, which is measured by custom made organ chips with integrated gold electrodes17. Additionally, the BBB chip displays low paracellular permeability, responds to inflammatory cues at the organ level, expresses active efflux pumps, and exhibits predictive transport of soluble biomarkers and biopharmaceuticals. Notably, BBB chips generated from several individuals captures the expected functional differences between healthy individuals and patients with neurological diseases15.
The protocol detailed below describes a reliable, efficient, and reproducible method for the generation of human iPSC-based BBB chips under dynamic flow conditions. Guidance is provided on the type of assays and endpoint analyses that can be performed directly in the BBB chip or from sampling effluent. Thus, the protocol demonstrates the spectrum of techniques that can be applied for evaluating biological and functional properties and responses in a human-relevant model.
A brief description of the iPSC-based BBB chip is provided here. Human iPSCs are initially differentiated and propagated in tissue culture flasks as free-floating aggregates of neural progenitors, termed EZ-spheres. The top channel of the Chip-S116,18,19 is seeded with dissociated EZ-spheres that form the "brain side" of the chip, as cells differentiate over 7 days into a mixed culture of neural progenitor cells (iNPCs), iAstrocytes, and iNeurons. Human iPSCs are also differentiated in tissue culture plates into iBMECs. The bottom channel of the chip is seeded with iBMECs to form the "blood side" as they develop to form an endothelial tube (Figure 1). The porous extracellular matrix (ECM)-coated membrane that separates the top and bottom channels 1) permits the formation of cell-to-cell interactions between channels and 2) allows the user to run permeability assays and image cells in either channel using a conventional light microscope.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Generation of iPSC-derived neural progenitor cells (iNPCs)
- Produce EZ-spheres from iPSC colonies as described below and as previously published20,21,22.
- Culture iPSC colonies to confluency on basement membrane matrix-coated 6 well plates (0.5 mg/plate) in mTESR1 or other commercial media (see Table of Materials).
- Remove iPSC medium and replace with 2 mL of EZ-sphere medium [ESM; DMEM:F12 7:3 supplemented with 100 ng/mL basic fibroblast growth factor (bFGF), 100 ng/mL epidermal growth factor, 5 µg/mL heparin, and 2% B27 supplement].
- Scrape the bottom of each confluent well with the back of a sterile 1000 µL pipette tip or cell scraper.
- Collect all cells and place in an ultra-low attachment T25 flask to allow the spontaneous formation of free-floating spheres. Incubate overnight at 37 °C.
- Feed the spheres every 2–3 days when the medium turns yellow by replacing half of the medium with fresh ESM. This allows for spheres to remain in conditioned medium, which is highly important for their growth and maintenance.
- Lean the flask on a tube rack and allow the spheres to settle by gravity for 1–2 min down to the corner of the flask.
- Once settled, aspirate half of the supernatant with a 5 mL or 10 mL serological pipette and replace with fresh, pre-warmed ESM.
- Passage EZ-spheres weekly by chopping spheres to 200 μm diameters as previously described23,20,21. EZ-spheres can be maintained for up to 25 passages and are ideal when used between passages 8–25.
- Prepare single cell suspension of iNPCs:
- To induce neural differentiation, dissociate EZ-sphere into single cells.
- Collect spheres 3–4 days post-chopping from a T75 flask and transfer into a 15 mL conical, then let it stand for 2 min or until all spheres are settled at the bottom.
- Slowly remove ESM with a 5 mL pipette without disrupting the settled spheres. Add 1 mL of dissociation solution (see Table of Materials) and incubate for 10 min at 37 °C.
- Swirl the dissociation solution and spheres after 5 min of incubation to ensure that any settled spheres are treated.
- Slowly remove the dissociation solution. Add 1 mL of neural differentiation medium [NDM; DMEM: F12 with 2% B27 minus vitamin A, 1% N2 supplement, and human brain-derived neurotrophic factor (hBDNF, 20 ng/mL)].
- Triturate the spheres into single cells by pipetting using a 1 mL pipette followed by 200 µL pipette, until all spheres have dissociated. Avoid bubble formation during the trituration procedure.
- Count dissociated cells using a hemocytometer and dilute cells to a final density of 1 x 106 cells/mL. It is possible to change the density depending on the application.
NOTE: Higher densities (up to 6 x 106 cells/mL) are recommended for short-term cultures of up to 3 days, and lower densities are recommended for long-term applications of up to 3 weeks.
NOTE: Cells are now ready to seed into the top channel of the chip to form the "brain side".
2. Differentiation of iPSCs into iBMECs
- Passage iPSCs from a single confluent well of a 6 well plate at a 1:6 ratio into a 6 well basement membrane matrix-coated plate. Let cells adhere for 24 h. Change iPSC medium daily.
- Count cells daily using a hemocytometer.
- When cells reach a density of 1.5–3.0 x 105 cells/well, replace iPSC medium with 3 mL of unconditioned medium without bFGF [DMEM:F12 1:1, with 10% knockout serum replacement (KOSR), 1% non-essential amino acids (NEAA), 0.5% glutamine supplement (Table of Materials), and 100 µM β-mercaptoethanol]. Replace medium daily for 6 days24.
- At day 6, replace medium with endothelial cell (EC) medium [human endothelial serum free medium (hESFM) supplemented with 1% platelet-poor plasma-derived bovine serum, 20 ng/mL bFGF, and 10 µM all-trans retinoic acid (RA)]. Leave medium for 2 days.
- Remove EC medium and add 1 mL of dissociation solution per well. Incubate at 37 °C for 35 min.
NOTE: While this is considered a long incubation time in the dissociation solution used here, iBMECs can endure this treatment with cell viability higher than 90%. - Detach the cells from the well by gently pipetting the cell suspension and collecting all cells into a 15 mL conical tube.
NOTE: Avoid harsh pipetting. If cells do not detach easily, incubate for additional 5 min. - Add 1 volume of EC medium into the 15 mL conical to inactivate Accutase, centrifuge at 200 x g for 5 min, remove medium, and replace with 1 mL of EC medium (without bFGF and RA).
- Count cells using a hemocytometer and adjust cell density to 14–20 x 106 cells/mL.
NOTE: Cells are now ready to be seeded into the bottom channel of the chip to form the "blood side".
3. Microfabrication of the organ chip
- Use an organ chip for the BBB chip model and its production as done previously16,18,19. The tall channel organ chip (see Table of Materials) is fabricated from a highly flexible polydimethylsiloxane (PDMS) elastomer that contains two superimposed and parallel micro-scale channels separated by a flexible porous membrane. The top and bottom microchannel sizes are 1 mm x 1 mm and 1.0 mm x 0.2 mm, respectively. The two channels are separated by a 50 μm thick PDMS-made flexible porous membrane, containing 7 μm diameter pores with 40 μm spacing. The surface area of the porous membrane that separates the channels is 0.171 cm2.
4. Chip preparation
- Organ chips are supplied prepackaged within the chip carrier, eliminating the need to disturb or distort the chip alignment during handling. In addition, the chip carrier connects securely to a portable module ("Pod") that acts as the interface between the culture module and chip.
- Spray the packaging of the chips with 70% ethanol and bring into the biosafety cabinet (BSC).
- Open the packaging and lay out the organ chip in a sterile Petri dish. Handle the chip carrier only by the sides to avoid direct contact with the chip.
- Ensure that the tab of the carrier is facing to the right (Figure 2), and when using multiple chips, align them all in the same orientation.
- Label each chip on the carrier tab (complete chip preparation and workflow is shown in Figure 3).
5. Surface activation and ECM coating
- Preparation of surface activation solution
- Emulate reagent 1 (ER-1), provided in a vial containing 5 mg, is light-sensitive. Prepare fresh ER-1 solution immediately before use. ER-1 integrity is crucial in successful preparation of the chips.
- Turn off the light in the BSC when handling ER-1.
NOTE: ER-1 is an eye irritant and must be handled in the BSC with proper gloves and eye protection. - Allow the ER-1 and ER-2 reagents to equilibrate to room temperature (RT) before use.
- Protect solution from light by wrapping an empty sterile 15 mL conical tube with foil.
- In the BSC, briefly tap the ER-1 vial to settle the powder at the bottom.
- Add 1 mL of ER-2 buffer to the vial, and immediately transfer its content to the bottom of the 15 mL wrapped conical tube. Do not pipette to mix. The color of the solution transferred to the conical tube will be red.
- Repeat step 5.1.6 3x. On the last round, cap the ER-1 vial and invert to collect any remaining powder from the lid, then transfer the solution to the conical tube; this will bring the total volume to 4 mL of ER-1 solution.
- Add 6 mL of ER-2 solution to the 4 mL of ER-1 solution in the 15 mL conical tube to a final concentration of 0.5 mg/mL. ER-1 should be fully dissolved within the ER-2 solution.
- Surface activation
- Utilizing a P200 pipette and a sterile 200 μL filtered pipette tip, take up 200 μL of ER-1 mixture.
- Place the pipette in the bottom inlet and push 20 μL of ER-1 mixture through the bottom channel until the mixture starts to flow out of the bottom channel outlet.
- Add approximately 50 μL of ER-1 mixture and place it in the top channel inlet. Push the mixture through the top channel until it starts flow out of the top channel outlet.
- Remove all excess ER-1 mixture from the surface of the chip by gentle aspiration. Make certain the ER-1 mixture is only removed from the chip surface and not from the channels.
- Verify that the channels are free of air bubbles before ultraviolet (UV) activation. If air bubbles are detected, remove bubbles by washing the channel with ER-1 mixture.
- Place the open dish containing the chips into the UV light box (provided by Emulate Inc.).
- Set the switch at the back of the UV light box to the "Consistent" setting. Turn on the power and initiate UV activation. Leave the chips under UV light for 20 min.
NOTE: Avoid exposure of personnel to UV light. - Remove the ER-1 mixture from both channels.
- Wash each channel with 200 μL of ER-2 solution.
- Remove ER-2 from both channels.
- Wash each channel with 200 μL of sterile cold Dulbecco's phosphate-buffered saline (DPBS).
- Leave cold DPBS inside the channels until proceeding to the next step.
- Extracellular matrix (ECM) preparation and coating
- Prepare the ECM solution by combining the individual ECM components with cold DPBS, water, or other solvent to the final working concentrations. The ECM solution should be prepared fresh each time it is used.
- Coat both top and bottom channels of the chip with ECM, with composition as determined by the cell type to be seeded. ECM mixture must be maintained on ice until use.
- Use laminin (50 µg/mL) to coat the "brain side", and collagen IV and fibronectin mixed at a 4:1 ratio (320:80 µg/mL) to coat the "blood side" as described in section 5.6.
- Preparation of ECM aliquots
- Dilute 1 mg/mL laminin in cold DPBS to a final concentration of 50 µg/mL. Aliquot and store at -20 °C until use.
- Dissolve collagen IV in 0.1% acetic acid to a concentration of 1 mg/mL. Incubate the solution at 2–8 °C overnight or at RT for 1-3 h or until fully dissolved.
- Prepare a 1 mL mixture of collagen IV:fibronectin (320 µL of collagen IV, 80 µL of fibronectin, 600 µL of sterile double-distilled H2O). The mixture can be stored at -20 °C.
- Coating the chips with ECM
- Fully aspirate the cold DPBS from both channels. Set a P200 pipette to take up 100 μL of collagen IV:fibronectin solution.
- Introduce the solution through the bottom channel inlet until a small droplet forms on the outlet. Leave a small droplet on the inlet after removing the pipette tip.
- Introduce the laminin solution through the top channel inlet until a small droplet forms on the outlet. Leave a small droplet on the inlet after removing the pipette tip.
- Look closely at the channels to ensure that no bubbles are present. If bubbles are present, wash the channel with the appropriate ECM solution until all bubbles are removed.
- Repeat steps 5.5.1–5.5.4 for each chip.
- Add 1.5 mL of DPBS to the cap of a 15 mL conical tube. Place the DPBS cap in the 150 mm culture dish with the chips to provide extra humidity and seal the dish with parafilm. For best results, incubate the chips at 4 °C overnight.
NOTE: If desired, cells can be seeded the same day as chip activation and ECM coating, though overnight incubation is preferred. Chips can be ready for seeding 4 h after adding the ECM and incubating chips at 37 °C.
6. Seeding the "brain side" channel and differentiating EZ spheres into mixed neural cultures
- Bring the dish containing the prepared chips to the BSC. Gently wash both channels with 200 µL of NDM.
- Avoiding contact with the ports, carefully aspirate excess media droplets from the surface of the chip. Gently agitate cell suspension before seeding each chip to ensure a homogeneous cell suspension
- Seeding the iNPCs into the top channel to generate the "brain side"
- Seed the cells (1 x 106 cells/mL) into the top channel of the chip. Add a P200 tip containing 30–100 μL of cells suspension to the top channel inlet and gently release the tip from the pipette. Take an empty P200 pipette, depress the plunger, insert into the top channel outlet and carefully pull the single cells suspension through the chip.
- Cover the dish and transfer to the microscope to check the seeding density and homogenous distribution of cells within the top channel. Gently remove the pipette tip from the chip inlet and outlet ports.
- Seeding density should appear as 20% coverage. If seeding density is higher or lower than expected or uneven, return the chips to the BSC, wash the channel 2x with 200 μL of fresh medium, and repeat step 6.3.1.
- After confirming the correct cell density, immediately place the chips in the incubator for 2 h at 37 °C after seeding each batch of chips. Wash away the cells that do not attach with fresh NDM.
- Keep cells under static conditions at 37 °C with a daily NDM replacement for at least 48 h before initiating flow. iBMECs can be seeded after iNPCs have attached or on a subsequent day following iNPC seeding.
7. Seeding iBMECs into the bottom channel to generate the "blood side"
- Bring the dish containing the prepared chips to the BSC. Gently wash the bottom channel with 200 µL of EC medium.
- Avoiding contact with the ports, carefully aspirate droplets of excess EC medium from the surface of the chip, making sure to leave medium in both channels. Gently agitate cell suspension before seeding each chip to ensure a homogeneous cell suspension.
- Using a P200 pipette, draw up 30–100 µL of the iBMEC cell suspension (14–20 x 106 cells/mL) and place the tip into the bottom channel inlet. Gently disconnect the tip from the pipette, leaving the cell-containing tip in the inlet port.
- Depress the plunger on a P200 pipette with an empty tip, insert into the bottom channel outlet, and carefully pull the single cell suspension through the bottom channel by slowly releasing the pipette plunger.
- Aspirate excess cell suspension from the surface of the chip. Avoid direct contact with the inlet and outlet ports to ensure that no cell suspension is aspirated out of the channels.
- Cover the chip and transfer it to the microscope to observe seeding density. The bottom channel should be filled with no observable gaps between cells when observed at 4x or 10x under a microscope (Figure 4).
- If seeding density is below 90% coverage or is unevenly distributed, adjust cell density accordingly and repeat steps 7.2–7.6 until the correct density is achieved within the channel. After confirming the correct cell density (Figure 4), seed cells in the remaining chips. To attach cells onto the porous membrane, which is located on the top of the bottom channel, invert each chip and rest in a chip cradle.
- Place a small reservoir (15 mL conical tube cap containing sterile DPBS) inside the 150 mm dish to provide humidity for the cells. Incubate chips at 37 °C for approximately 3 h, or until cells in the bottom channel have attached. Once iBMECs have attached (~3 h post-seeding), flip the chips back to an upright position to allow cell attachment to the bottom portion of the bottom channel.
8. Initiation of flow
- Flow is typically initiated 48 h post-seeding of iBMECs. This time is required for the iBMECs to attach firmly to the chip.
- To maintain laminar flow through the chip, it is important to degas and equilibrate the temperature of the medium. Medium must be pre-warmed in a 37 °C water bath for 1 h.
- Up to 50 mL of warmed medium can be degassed by incubation under a vacuum-driven filtration system for 15 min.
- Priming of portable modules
- Sanitize the exterior of the portable module packaging and trays with 70% ethanol, wipe, and transfer to the BSC. Open the package and place the modules into the tray. Orient them with the reservoirs toward the back of the tray.
- Pipette 3 mL of pre-equilibrated, warm media to each inlet reservoir. Add EC culture medium to the inlet reservoir of the bottom channel and NDM to the top channel inlet reservoir of the top channel (Figure 5).
- Pipette 300 μL of pre-equilibrated, warm media to each outlet reservoir, directly over each outlet port (Figure 5).
- Place up to six portable modules on each tray. Bring trays to the incubator and slide completely into the culture module with the tray handle facing outward.
- Select and run the "Prime" cycle on the culture module. Close the incubator door and allow the culture module to prime the portable modules (takes ~1 min). The priming cycle is completed when the status bar reads "Ready". Remove the tray from the culture module and bring it to the BSC.
- Verify that the portable modules were successfully primed by inspecting the underside of each portable module in the BSC. Look for the presence of small droplets at all four ports.
- If any portable module does not show droplets, rerun the prime cycle on those modules. If any media dripped onto the tray (this may occur more often by the outlet ports), clean tray with 70% ethanol.
- Connection of chips to portable modules, regulation, and initiation of flow
- Gently wash both channels of each chip with warm, equilibrated cell-specific culture medium to remove any possible bubbles in the channel and place small droplets of media (according to the media in the channel) on the top of each inlet and outlet port.
- Insert chips with carriers into the portable modules and place up to six on each tray. Insert trays into the culture module. Program the appropriate organ chip culture conditions (flow rate and stretch) on the culture module.
- Programmed conditions will start as soon as the "Regulate" cycle is complete.
NOTE: The flow rates for each channel can be controlled independently and can be set to rates that range from 0–1,000 µL/h. The BBB chip is typically cultured at 30 µL/h. When flowing media such as EC media or ESM at 30 µL/h and 1000 µL/h, the shear forces are 0.01 dyn/cm2 and 0.33 dyn/cm2, respectively. - Run the "Regulate" cycle, which takes approximately 2 h, after which the culture module will begin flow at the preset organ chip culture conditions.
9. Blood-to-brain paracellular permeability assessment
- Prepare NDM supplemented with 10 µg/mL dextran-FITC (4 kDa). This solution will be used as the input for the "blood side" channel.
- Fill the bottom channel reservoirs of the portable modules with NDM supplemented with dextran-FITC. Fill the top channel reservoirs with NDM without tracer.
- Perfuse both, the top and bottom channels at a flow rate of 30 µL/h for at least 4 h until enough media accumulates to be collected for assessment of fluorescence in a plate reader (typically 100 µL).
- Collect media samples from the input and output reservoirs of the top and bottom channels. Protect the samples from light.
- Serially dilute the NDM supplemented with 10 µg/mL dextran-FITC 1:1 using NDM without tracer to generate a 10–12 point calibration curve.
- Take 100 µL of each sample, including the calibration curve, into a black 96 well plate and read fluorescence using a plate reader (485 nm excitation, 530 nm emission).
- Use the measured values to compute Papp values as follows:
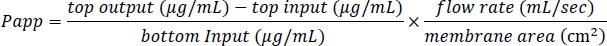
- Take daily measurements to assess the barrier properties and confirm that the BBB chip is still functional.
10. Immunocytochemistry
- Bring chips to a chemical fume hood. Using a P200 pipette, fix cells by perfusing both channels with 200 µL of 4% paraformaldehyde (PFA) in DPBS and incubate for 10 min at RT.
- Following the fixation, perfuse each channel with 200 μL of DPBS and incubate for 5 min. Repeat DPBS washing 2x.
- Block and permeabilize cells on the chip by perfusing primary blocking solution (PBS, supplemented with 5% normal donkey serum and 0.1% Triton X-100). Incubate at RT for 1 h.
- Dilute primary antibodies in primary blocking solution and incubate overnight at 4 °C. BMECs markers are GLUT-1 (1:100 dilution), ZO-1 (1:300), PECAM-1 (1:250; CD31), and VE-cadherin (1:200). Neural markers are βIII-tubulin (1:1000; Tuj1α), S100β (1:500), nestin (1:1000) and GFAP (1:1000).
- Wash the chips 3x with cold DPBS.
- Dilute secondary antibodies in secondary blocking solution (DPBS with 5% normal donkey serum without Triton-X).
- Perfuse the secondary antibody solution through both channels. Typically, fluorescent secondary antibodies are diluted 1:1000. Incubate for 1 h at RT protected from light.
- Wash the chips 3x with DPBS.
- Stain cell nuclei by perfusing chip with 100 μL of DAPI solution. Incubate at RT for 5 min.
- Wash the chips 3x with DPBS.
- The chip is ready for imaging using either an upright or an inverted fluorescent microscope. The PDMS transparency permits imaging in the intact organ chip. Magnifications above 10x may require long working distance objectives due to the thickness of the organ chip.
Access restricted. Please log in or start a trial to view this content.
Results
Figure 6A,B,C represents a BBB chip seeded with EZ-spheres on the "brain side" top channel and iBMECs on the "blood side'" bottom channel. iBMECs were seeded first and allowed to attach overnight, after which EZ-spheres were seeded. Chips were then cultured under static conditions with daily media replacement for seven days. The BBB chip was then fixed using 4% PFA at RT for 10 min and washed 3x with DPBS. Immunocytochemistry was performed on the BBB chip...
Access restricted. Please log in or start a trial to view this content.
Discussion
The combination of organ-on-chip technology and iPSC-derived cells in the NVU holds promise for accurate modeling of the human BBB. Here, we provide a detailed protocol for simple and robust application of the recently published iPSC-based BBB chip16. An overview and timing of the seeding paradigm is shown in Figure 3. To obtain and maintain barrier functions that are suitable for BBB modeling, generating a homogenous iBMEC monolayer and retaining its integrity are cr...
Access restricted. Please log in or start a trial to view this content.
Disclosures
Cedars-Sinai owns a minority stock interest in Emulate, the company that produces the study's microfluidic Organ chips. An officer of Cedars-Sinai also serves on Emulate's Board of Directors. Emulate provided no financial support for this research. Cedars-Sinai and Emulate have patents filed related to this work.
Acknowledgements
We would like to thank Dr. Soshana Svendsen for critical editing. This work was supported by the Israel Science Foundation grant 1621/18, the Ministry of Science and Technology (MOST), Israel 3-15647, the California Institute for Regenerative Medicine grant ID DISC1-08800, the Sherman Family Foundation, NIH-NINDS grant 1UG3NS105703, and The ALS Association grant 18-SI-389. AH was funded by Wallenberg Foundation (grant number 2015.0178).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Accutase | EMD Millipore | SCR005 | Dissociation solution |
| B27 | Gibco | 12587010 | |
| Bfgf | Peprotech | 100-18B | |
| Chip-S1 | Emulate Inc | Chip-S1 | Organ-Chip |
| Collagen IV | Sigma | C5533 | |
| DAPI | Invitrogen | D3571 | |
| Dextran-FITC | Sigma | 46944 | |
| DMEM: F12 | Thermo Fisher Scientific | 31330038 | |
| Donkey serum | Sigma | D9663 | |
| Emulate Reagent 1 (ER-1) | Emulate Inc | ER-1 | |
| Emulate Reagent 2 (ER-2) | Emulate Inc | ER-2 | |
| Fibronectin | Sigma | F1141 | |
| Glial Fibrillary Acidic Protein (GFAP) | Dako | Z0334 | |
| GLUT-1 | Invitrogen | MA5-11315 | |
| Glutamax | Life Technologies | 35050038 | Glutamine supplement |
| hBDNF | Peprotech | 450-02 | |
| KOSR | Thermo Fisher Scientific | 10828028 | |
| Laminin | Sigma | L2020 | |
| Matrigel | Corning | 354234 | Basement membrane matrix |
| mTeSR1 | StemCell Technologies, Inc. | 85851 | |
| NEAA | Biological industries | 01-340-1B | |
| Nestin | Millipore | MAB353 | |
| NutriStem | Biological industries | 05-100-1A | Alternate media |
| PECAM-1 | Thermo Fisher Scientific | 10333 | |
| Platelet-poor plasma-derived bovine serum (PPP) | Biomedical Technologies | J64483AB | |
| Retinoic acid (RA) | Sigma | R2625 | |
| S100β | Abcam | ab6602 | |
| Steriflip-GP Sterile Centrifuge Tube Top Filter Unit | Millipore | SCGP00525 | |
| Triton X-100 | Sigma | X100 | |
| ZO-1 Monoclonal Antibody | Invitrogen | 33-9100 | |
| βIII-tubulin (Tuj1α) | Sigma | T8660 | |
| β-mercaptoethanol | Life Technologies | 31350010 |
References
- Pardridge, W. M. Blood-brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opinion on Therapeutic Targets. 19 (8), 1059-1072 (2015).
- Gastfriend, B. D., Palecek, S. P., Shusta, E. V. Modeling the blood-brain barrier: beyond the endothelial cells. Current Opinion in Biomedical Engineering. 5, 6-12 (2018).
- Jamieson, J. J., Linville, R. M., Ding, Y. Y., Gerecht, S., Searson, P. C. Role of iPSC-derived pericytes on barrier function of iPSC-derived brain microvascular endothelial cells in 2D and 3D. Fluids and Barriers of the CNS. 16 (1), 15(2019).
- El-Habashy, S. E., et al. Novel treatment strategies for brain tumors and metastases. Pharmaceutical Patent Analyst. 3 (3), 279-296 (2014).
- Lim, R. G., et al. Huntington's disease iPSC-derived brain microvascular endothelial cells reveal WNT-mediated angiogenic and blood-brain barrier deficits. Cell Reports. 19 (7), 1365-1377 (2017).
- Dumitrescu, A. M., Liao, X. H., Weiss, R. E., Millen, K., Refetoff, S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 147 (9), 4036-4043 (2006).
- Spencer, J. I., Bell, J. S., DeLuca, G. C. Vascular pathology in multiple sclerosis: reframing pathogenesis around the blood-brain barrier. Journal of Neurology and Neurosurgical Psychiatry. 89 (1), 42-52 (2018).
- Yamazaki, Y., Kanekiyo, T. Blood-brain barrier dysfunction and the pathogenesis of Alzheimer's disease. International Journal of Molecular Sciences. 18 (9), 1965(2017).
- Ben-Zvi, A., et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 509 (7501), 507(2014).
- Heng, M. Y., Detloff, P. J., Albin, R. L. Rodent genetic models of Huntington disease. Neurobiology of Disease. 32 (1), 1-9 (2008).
- Ho, R., et al. ALS disrupts spinal motor neuron maturation and aging pathways within gene co-expression networks. Nature Neuroscience. 19 (9), 1256(2016).
- Griep, L. M., et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomedical Microdevices. 15 (1), 145-150 (2013).
- Prabhakarpandian, B., et al. Synthetic tumor networks for screening drug delivery systems. Journal of controlled release. 201, 49-55 (2015).
- Delsing, L., et al. Barrier properties and transcriptome expression in human iPSC-derived models of the blood-brain barrier. Stem Cells. 36 (12), 1816-1827 (2018).
- Park, T. E., et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nature Communications. 10 (1), 2621(2019).
- Vatine, G. D., et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell. 24 (6), 995-1005 (2019).
- Henry, O. Y. F., Villenave, R., Cronce, M. J., Leineweber, W. D., Benz, M. A., Ingber, D. E. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab on a Chip. 17 (13), 2264-2271 (2017).
- Sances, S., et al. Human iPSC-derived endothelial cells and microengineered organ chip enhance neuronal development. Stem Cell Reports. 10 (4), 1222-1236 (2018).
- Workman, M. J., et al. Enhanced utilization of induced pluripotent stem cell–derived human intestinal organoids using microengineered chips. Cellular and Molecular Gastroenterology and Hepatology. 5 (4), 669-677 (2018).
- Ebert, A. D., et al. EZ spheres: a stable and expandable culture system for the generation of pre-rosette multipotent stem cells from human ESCs and iPSCs. Stem Cell Research. 10 (3), 417-427 (2013).
- Shelley, B. C., Gowing, G., Svendsen, C. N. A cGMP-applicable expansion method for aggregates of human neural stem and progenitor cells derived from pluripotent stem cells or fetal brain tissue. Journal of Visualized Experiments. (88), e51219(2014).
- Vatine, G. D., et al. Modeling psychomotor retardation using iPSCs from MCT8-deficient patients indicates a prominent role for the blood-brain barrier. Cell Stem Cell. 20 (6), 831-843 (2017).
- Svendsen, C. N., et al. A new method for the rapid and long term growth of human neural precursor cells. Journal of Neuroscience Methods. 85 (2), 141-152 (1998).
- Lippmann, E. S., Al-Ahmad, A., Azarin, S. M., Palecek, S. P., Shusta, E. V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Scientific Reports. 4, 4160(2014).
- Canfield, S. G., et al. An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. Journal of Neurochemistry. 140 (6), 874-888 (2017).
- Jamieson, J. J., Gerecht, S. Chipping Away at Blood-Brain-Barrier Modeling. Cell stem cell. 24 (6), 831-832 (2019).
- Faal, T., et al. Induction of Mesoderm and Neural Crest-Derived Pericytes from Human Pluripotent Stem Cells to Study Blood-Brain Barrier Interactions. Stem Cell Reports. 12 (3), 451-460 (2019).
- Lippmann, E. S., et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nature Biotechnology. 30 (8), 783(2012).
- Qian, T., et al. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Science Advances. 3 (11), 1701679(2017).
- Neal, E. H., et al. A Simplified, Fully Defined Differentiation Scheme for Producing Blood-Brain Barrier Endothelial Cells from Human iPSCs. Stem Cell Reports. 12 (6), 1380-1388 (2019).
- Wevers, N. R., et al. A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids and Barriers of the CNS. 15 (1), 23(2018).
- Huh, D., et al. Microfabrication of human organs-on-chips. Nature Protocols. 8 (11), 2135(2013).
- Huh, D., Matthews, B. D., Mammoto, A., Montoya-Zavala, M., Hsin, H. Y., Ingber, D. E. Reconstituting organ-level lung functions on a chip. Science. 328 (5986), 1662-1668 (2010).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved