A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Assessment of Cellular Oxidation using a Subcellular Compartment-Specific Redox-Sensitive Green Fluorescent Protein
In This Article
Summary
This protocol describes the assessment of subcellular compartment-specific redox status within the cell. A redox-sensitive fluorescent probe allows convenient ratiometric analysis in intact cells.
Abstract
Measuring the intracellular oxidation/reduction balance provides an overview of the physiological and/or pathophysiological redox status of an organism. Thiols are especially important for illuminating the redox status of cells via their reduced dithiol and oxidized disulfide ratios. Engineered cysteine-containing fluorescent proteins open a new era for redox-sensitive biosensors. One of them, redox-sensitive green fluorescent protein (roGFP), can easily be introduced into cells with adenoviral transduction, allowing the redox status of subcellular compartments to be evaluated without disrupting cellular processes. Reduced cysteines and oxidized cystines of roGFP have excitation maxima at 488 nm and 405 nm, respectively, with emission at 525 nm. Assessing the ratios of these reduced and oxidized forms allows the convenient calculation of redox balance within the cell. In this method article, immortalized human triple-negative breast cancer cells (MDA-MB-231) were used to assess redox status within the living cell. The protocol steps include MDA-MB-231 cell line transduction with adenovirus to express cytosolic roGFP, treatment with H2O2, and assessment of cysteine and cystine ratio with both flow cytometry and fluorescence microscopy.
Introduction
Oxidative stress was defined in 1985 by Helmut Sies as “a disturbance in prooxidant–antioxidant balance in favor of the former”1, and a plethora of research has been conducted to obtain disease-, nutrition-, and aging-specific redox status of organisms1,2,3. Since then, the understanding of oxidative stress has become broader. Testing the hypotheses of using antioxidants against diseases and/or aging has shown that oxidative stress not only causes harm but also has other roles in cells. Furthermore, scientists have shown that free radicals play an important role for signal transduction2. All of these studies strengthen the importance of determining the changes in reduction-oxidation (redox) ratio of macromolecules. Enzyme activity, antioxidants and/or oxidants, and oxidation products can be assessed with various methods. Among these, methods that determine thiol oxidation are arguably the most used because they report on the balance between antioxidants and prooxidants in cells, as well as organisms4. Specifically, ratios between glutathione (GSH)/glutathione disulfide (GSSG) and/or cysteine (CyS)/cystine (CySS) are used as biomarkers for monitoring the redox status of organisms2.
Methods used for assaying the balance between prooxidants and antioxidants rely mainly on the levels of reduced/oxidized proteins or small molecules within cells. Western blots and mass spectrometry are used to broadly assess the ratios of reduced/oxidized macromolecules (protein, lipids etc.), and GSH/GSSG ratios can be assessed with spectrophotometry5. A common feature of these methods is the physical perturbation of the system by cell lysis and/or tissue homogenization. These analyses also become challenging when it is necessary to measure the oxidation status of different cellular compartments. All of these perturbations cause artifacts in the assay environment.
Redox-sensitive fluorescent proteins opened an advantageous era for evaluating the redox balance without causing a disturbance in the cells6. They can target different intracellular compartments, allowing the quantification of compartment-specific activities (e.g., assaying the redox state of mitochondria and the cytosol) to investigate crosstalk between cellular organelles. Yellow fluorescent protein (YFP), green fluorescent protein (GFP), and HyPeR proteins are reviewed by Meyer and colleagues6. Among these proteins, redox-sensitive GFP (roGFP) is unique due to different fluorescent readouts of its CyS (ex. 488 nm/em. 525 nm) and CySS (ex. 405 nm/525 nm) residues, which permits ratiometric analysis, unlike other redox-sensitive proteins such as YFP7,8. Ratiometric output is valuable because it counterbalances the differences between expression levels, detection sensitivities, and photobleaching8. Subcellular compartments of cells (cytosol, mitochondria, nucleus) or different organisms (bacteria as well as mammalian cells) can be targeted by modifying roGFP7,9,10.
roGFP assays are conducted using fluorescent imaging techniques, especially for real-time visualization experiments. Flow cytometric analyses of roGFPs are also possible for experiments with predetermined time points. The current article describes both the use of fluorescent microscopy and flow cytometry to perform a ratiometric assessment of redox status in mammalian cells overexpressing roGFP (targeted to cytosol) via adenoviral transduction.
Access restricted. Please log in or start a trial to view this content.
Protocol
NOTE: This protocol was optimized for 70%–80% confluent MDA-MB-231 cells. For other cell lines, the number of cells and multiplicity of infection (MOI) should be reoptimized.
1. Preparation of cells (day 1)
- Maintain MDA-MB-231 cell line in 75 cm2 flasks with 10 mL of Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a 5% CO2 humidified atmosphere.
NOTE: DMEM supplemented with 10% FBS, 37 °C, and a 5% CO2 humidified atmosphere are used for all attachment and treatment incubations throughout the entire protocol. - Prepare the MDA-MB-231 cells for experiment.
- Aspirate the medium within the flask, detach the cells with 2 mL of 0.25% trypsin-EDTA solution for 2 min, and inactivate the trypsin activity with 6 mL of complete medium (DMEM with 10% FBS). Centrifuge the cells at 150 x g for 5 min. Aspirate the supernatant and suspend the cells in 5 mL of complete medium.
- Mix an equal volume cell suspension and 0.4% trypan blue. Take 10 µL of this mixture and count the cells with the automated cell counter.
NOTE: A Coulter counter or a hemocytometer can also be used for cell counting. - Seed the cells into a 6 well plate for flow cytometry analyses and seed 150,000 cells in 1 mL of medium per well. Wait 16 h for cell attachment.
- Seed the cells into a 4 well chamber slide for fluorescent imaging and seed 25,000 cells in 0.5 mL of medium per well. Wait 16 h for cell attachment.
NOTE: Seed control wells in addition to treatment wells. Use one of the control wells to determine cell number (optional: if the attachment period for the cells is shorter than the doubling time, cell number can be assumed to be the same as the seeding density) and the other for a noninfected control (0 MOI).
2. Adenoviral roGFP transduction (day 2 and 3)
CAUTION: Adenoviruses can cause diseases. While transducing the cells, use filtered tips and decontaminate tips, Pasteur pipettes, and microcentrifuge tubes with 10% bleach.
NOTE: This protocol was demonstrated with cytosol-specific roGFP, but other cellular compartments (e.g., mitochondria or mitochondrial intermembrane space) can be targeted with this same protocol.
- Generate a dose-response curve for the MOI to obtain the highest transduction efficiency by calculating the volume of adenovirus (mL) required for each MOI value for MDA-MB-231 cell line (Table 1):
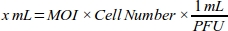
NOTE: Functional titer of each batch of adenoviral stock, which is expressed as plaque forming unit (PFU) per mL, is provided by the company. The optimum MOI for transduction differs between cell types. For most mammalian cells, the optimum MOI range is between 10 and 300. According to the cellular response, MOI values should be recalculated (e.g., MOI range should be reduced if cells have cytotoxic response, or range should be increased if cells have low transduction efficiency). - Make 1:100 dilution of 6 x 1010 PFU/mL adenoviral roGFP solution with cell culture medium (DMEM with 10% FBS) for reliable pipetting.
- Pipette and add 0.0125 mL (12.5 µL), 0.025 mL (25 µL), 0.05 mL (50 µL) of adenoviral roGFP dilution into each well of the 6 well plate in order to transduce the 150,000 cells with 50, 100, and 200 MOI respectively for flow cytometry analysis (Table 1).
- Pipette and add 0.0042 mL (4.2 µL) of adenoviral roGFP dilution in the 4-chamber slide wells to transduce 25,000 cells with 100 MOI for fluorescence imaging (Table 1).
NOTE: A minimal amount of medium should be used in the wells to ensure the highest interaction between the adenoviral roGFP construct and cells. The serum content of the culture medium may need to be decreased for different cell lines because high levels of serum can negatively affect transduction efficiency in some cell types. - Incubate cells for 16–24 h under the cell maintenance conditions. The next day (day 3), change the medium to cell culture medium (DMEM with 10% FBS) to allow cell recovery for an additional 24 h. Visualize cells under a microscope to assess their morphology; cells can express roGFP even if they have morphological changes.
NOTE: On day 3, cells should start to express roGFP; therefore, transduction efficiency can be monitored using fluorescence microscopy (filters with ex. 488/em. 525). To obtain consistent assay results, be aware of and document the morphological changes under the phase contrast microscope and observe morphology while evaluating transduction efficiency. - Construct a dose response curve using the 50, 100 and 200 MOI samples prepared in step 2.3 and their transduction efficiency results obtained from flow cytometry analysis (steps 3.1 and 4.1). Assess optimal transduction efficiency with documentation of morphological changes (step 2.5) and the dose-response curve of MOI.
NOTE: Although more than 98% of the cell population at 100 MOI and 200 MOI express roGFP (see representative results), 200 MOI group showed substantial changes in cell morphology of MDA-MB-231 cells. Consequently, the most efficacious MOI for MDA-MB-231 cells was determined to be 100 MOI. - After optimal MOI (here, 100 MOI) was chosen for MDA-MB-231 cell line, conduct experiment with test materials (10 µM H2O2 and its vehicle 0.1% deionized water).
- Prepare and seed the cells according to section 1. Using the adenoviral transduction volume for 100 MOI calculated in step 2.1, repeat steps 2.2−2.4 for 100 MOI adenoviral transduction of cells. Then incubate the plate and chamber slides according to step 2.5.
3. Acquisition of CyS/CySS balance
- Flow cytometry (day 4)
- On day 4, incubate cells from step 2.7.1 with 10 µM H2O2 for 1 h.
NOTE: 10 µM H2O2 was used as the test substance and 0.1% deionized water was used as vehicle treatment in this protocol. Other oxidizing agents can be used as positive controls here. - Aspirate media from the 6 well plate, replace with 750 µL of 0.25% trypsin-EDTA solution and wait for 2 min for cells to detach. Inactivate trypsin with 2 mL of complete medium (DMEM with 10% FBS) and collect the volume into 15 mL conical tubes.
- Centrifuge the tubes at 150 x g for 5 min at 4ºC. Discard supernatant and suspend the cells in 500 µL of phosphate-buffered saline (PBS).
- Repeat step 3.1.3
- Filter the cell suspensions into flow cytometry-compatible tubes using 40 µm mesh. Keep the tubes on ice and away from the light and follow step 4.1 for data analysis.
- On day 4, incubate cells from step 2.7.1 with 10 µM H2O2 for 1 h.
- Microscopic imaging (day 4)
- On day 4, treat cells with 10 µM H2O2, acquire images immediately (time point 0) and 1 h after treatment and follow step 4.2 for data analysis.
4. Data analysis
- Flow cytometry quantification
- Set flow cytometry method for 3 different analyses via sample aquisition software (see Table of Materials): forward scatter (FCS) on x-axis and side scatter (SSC) on y-axis to assess cell size and complexity of cells (SSC can be used for rough identification of dead and live cells); ex. 488 nm/em. 525 nm (fluorescein isothiocyanate [FITC]) bandpass filter on x-axis and SSC on y-axis to assess CyS-roGFP; ex. 405 nm/em. 525 nm (Brilliant Violet 510 [BV510]) bandpass filter on x-axis and SSC on y-axis to assess CySS-roGFP.
- Acquire 0 MOI control and visualize cells with sample acquisition software. Repeat this step for remaining samples (50, 100, 200 MOI groups and later on 10 µM H2O2 treated cells and vehicle treated cells). Save the files for data analysis.
- Open data analysis software (see Table of Materials) and open 0 MOI sample file. Assess cell population of interest (Gate 1). Set up the following gatings to minimize background fluorescence for ex. 488 nm/em. 525 nm (Gate 2) and ex. 405 nm/em. 525 nm (Gate 3) bandpass filters with the noninfected (0 MOI) control cells.
- Open 50, 100, and 200 MOI sample files within data analysis software to assess the dose-response curve. Analyze mean fluorescence intensities with Gates 2 and 3 for each sample. Repeat this step for test samples (10 µM H2O2 treated cells and vehicle treated cells).
- Calculate the mean fluorescent intensity ratio between oxidized versus reduced forms of roGFP with the following equation.

- Image assessment
- Use a microscope that contains fluorescence filters for CyS-roGFP and CySS-roGFP (ex. 488 nm/em. 525 nm and ex. 405 nm/em. 525 nm filters, respectively).
- In each well of the chamber slide, pick 4 random areas to acquire images, using the 4x objective to visualize larger areas.
NOTE: 20x objective can also be used for image displays. - Open the image with ImageJ software11. Apply Analyze | Measure commands for each image and use the equation in step 4.1.5 to quantify the data.
NOTE: Quantification of the images is ratiometric; therefore, the protocol does not include subtraction of background. However, to be able to compare images, brightness, contrast, and saturation must be the same for each image. Statistical significance was assessed with one-way analysis of variance (ANOVA) and Tukey’s post hoc test.
Access restricted. Please log in or start a trial to view this content.
Results
The redox state of CyS/CySS is easily assayed with transduced roGFPs. The fluorescent probe quantifies the ratio between the reduced and oxidized forms (excitation wavelengths 488 nm and 405 nm, respectively). Fluorescence data can be obtained by both flow cytometry and microscopy.
A large number of cells can consistently and conveniently be acquired using flow cytometry. The analysis consists of 3 main steps: 1) select the cell population of interest with the FSC area filter (
Access restricted. Please log in or start a trial to view this content.
Discussion
The thiol/disulfide balance in an organism reflects the redox status of cells. Living organisms have glutathione, cysteine, protein thiols, and low-molecular-weight thiols, all of which are affected by the level of oxidation and echo the redox status of cells4. Engineered roGFPs allow the non-disruptive quantification of the thiol/disulfide balance via their CyS residues7. The ratiometric property of roGFP provides reliable redox measurements for mammalian cells. roGFP can ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The construct and recombinant adenovirus for expressing cytosol-specific roGFP in cells were generated in the laboratory of Paul T. Schumacker, PhD, Freiberg School of Medicine, Northwestern University, and ViraQuest Inc., respectively. This study was supported by the Center for Studies of Host Response to Cancer Therapy grant P20GM109005 through the NIH National Institute of General Medical Sciences Centers of Biomedical Research Excellence (COBRE NIGMS), National Institute of General Medical Sciences Systems Pharmacology and Toxicology Training Program grant T32 GM106999, UAMS Foundation/Medical Research Endowment Award AWD00053956, UAMS Year-End Chancellor’s Awards AWD00053484. The flow cytometry core facility was supported in part by the Center for Microbial Pathogenesis and Host Inflammatory Responses grant P20GM103625 through the COBRE NIGMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. ATA was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) 2214-A scholarship.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 0.25% Trypsin-EDTA | Gibco by Life Sciences | 25200-056 | Cell culture |
| 4-well chamber slide | Thermo Scientific | 154526 | Cell seeding material for fluorescent imaging |
| 5 ml tubes with cell strainer cap | Falcon | 352235 | Single cell suspension tube for flow cytometry analysis |
| 6-well plate | Corning | 353046 | Cell seeding material for flow cytometry analysis |
| 15 ml conical tubes | MidSci | C15B | Cell culture |
| 75 cm2 ventilated cap tissue culture flasks | Corning | 4306414 | Cell culture |
| Adenoviral cytosol specific roGFP | ViraQuest | VQAd roGFP | roGFP construct kindly provided by Dr. Schumaker |
| Class II, Type A2 Safety Hood Cabinet | Thermo Scientific | 1300 Series A2 | Cell culture |
| Countess automated cell counter | Invitrogen | C10227 | Cell counting |
| Countess cell counter chamber slides | Invitrogen | C10283 | Cell counting |
| DMEM | Gibco by Life Sciences | 11995-065 | Cell culture |
| FBS | Atlanta Biologicals | S11150 | Cell culture |
| Filtered pipette tips, sterile, 20 µl | Fisherbrand | 02-717-161 | Cell culture |
| Filtered pipette tips, sterile, 1000 µl | Fisherbrand | 02-717-166 | Cell culture |
| Flow Cytometer | BD Biosciences | LSRFortessa | Instrument equipped with FITC and BV510 bandpass filters for flow cytometry analyses |
| Fluorescent Microscope | Advanced Microscopy Group (AMG) | Evos FL | Fluorescent imaging |
| Hydrogen Peroxide 30% | Fisher Scientific | H325-100 | Positive control |
| Light Cube, Custom | Life Sciences | CUB0037 | Fluorescent imaging of roGFP expressing cells (ex 405 nm) |
| Light Cube, GFP | Thermo Scientific | AMEP4651 | Fluorescent imaging of roGFP expressing cells (ex 488 nm) |
| MDA-MB-231 | American Tissue Culture Collection | HTB-26 | Human epithelial breast cancer cell line |
| Microcentrifuge tubes, 2 ml | Grenier Bio-One | 623201 | Cell culture |
| PBS | Gibco by Life Sciences | 10010-023 | Cell culture |
| Pipet controller | Drummond | Hood Mate Model 360 | Cell culture |
| Serologycal pipet, 1 ml | Fisherbrand | 13-678-11B | Cell culture |
| Serologycal pipet, 5 ml | Fisherbrand | 13-678-11D | Cell culture |
| Serologycal pipet, 10 ml | Fisherbrand | 13-678-11E | Cell culture |
| Tissue Culture Incubator | Thermo Scientific | HERACell 150i | CO2 incubator for cell culture |
| Trypan blue stain 0.4% | Invitrogen | T10282 | Cell counting |
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biology. 4, 180-183 (2015).
- Jones, D. P. Redefining Oxidative Stress. Antioxidants & Redox Signalling. 8 (9-10), (2006).
- Pizzino, G., et al. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity. 20117, 8416763(2017).
- Go, Y. M., Jones, D. P. Thiol/disulfide redox states in signaling and sensing. Critical Reviews in Biochemistry and Molecular Biology. 48 (2), 173-191 (2013).
- Hansen, J. M., Go, Y., Jones, D. P. Nuclear and Mitochondrial Compartmentation of Oxidative Stress and Redox Signaling. Annual Review of Pharmacology and Toxicology. 46 (1), 215-234 (2006).
- Meyer, A. J., Dick, T. P. Fluorescent protein-based redox probes. Antioxidants and Redox Signaling. 13 (5), 621-650 (2010).
- Dooley, C. T., et al. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. Journal of Biological Chemistry. 279 (21), 22284-22293 (2004).
- Björnberg, O., Østergaard, H., Winther, J. R. Measuring intracellular redox conditions using GFP-based sensors. Antioxidants and Redox Signaling. 8 (3-4), 354-361 (2006).
- Bhaskar, A., et al. Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of Mycobacterium tuberculosis during Infection. PLoS Pathogens. 10 (1), 1003902(2014).
- Loor, G., et al. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochimica et Biophysica Acta - Molecular Cell Research. 1813 (7), 1382-1394 (2011).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 9 (7), 671-675 (2012).
- Loor, G., et al. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radical Biology and Medicine. 49 (12), 1925-1936 (2010).
- Esposito, S., et al. Redox-sensitive GFP to monitor oxidative stress in neurodegenerative diseases. Reviews in the Neurosciences. 28 (2), 133-144 (2017).
- Meyer, A. J., et al. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant Journal. 52 (5), 973-986 (2007).
- Galvan, D. L., et al. Real-time in vivo mitochondrial redox assessment confirms enhanced mitochondrial reactive oxygen species in diabetic nephropathy. Kidney International. 92 (5), 1282-1287 (2017).
- Swain, L., Nanadikar, M. S., Borowik, S., Zieseniss, A., Katschinski, D. M. Transgenic organisms meet redox bioimaging: One step closer to physiology. Antioxidants and Redox Signaling. 29 (6), 603-612 (2018).
- Gutscher, M., et al. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. Journal of Biological Chemistry. 284 (46), 31532-31540 (2009).
- Morgan, B., Sobotta, M. C., Dick, T. P. Measuring EGSH and H2O2 with roGFP2-based redox probes. Free Radical Biology and Medicine. 51 (11), 1943-1951 (2011).
- Dey, S., Sidor, A., O'Rourke, B. Compartment-specific control of reactive oxygen species scavenging by antioxidant pathway enzymes. Journal of Biological Chemistry. 291 (21), 11185-11197 (2016).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved