Method Article
Manipulation of Single Neural Stem Cells and Neurons in Brain Slices using Robotic Microinjection
* These authors contributed equally
In This Article
Summary
This protocol demonstrates the use of a robotic platform for microinjection into single neural stem cells and neurons in brain slices. This technique is versatile and offers a method of tracking cells in tissue with high spatial resolution.
Abstract
A central question in developmental neurobiology is how neural stem and progenitor cells form the brain. To answer this question, one needs to label, manipulate, and follow single cells in the brain tissue with high resolution over time. This task is extremely challenging due to the complexity of tissues in the brain. We have recently developed a robot, that guide a microinjection needle into brain tissue upon utilizing images acquired from a microscope to deliver femtoliter volumes of solution into single cells. The robotic operation increases resulting an overall yield that is an order of magnitude greater than manual microinjection and allows for precise labeling and flexible manipulation of single cells in living tissue. With this, one can microinject hundreds of cells within a single organotypic slice. This article demonstrates the use of the microinjection robot for automated microinjection of neural progenitor cells and neurons in the brain tissue slices. More broadly, it can be used on any epithelial tissue featuring a surface that can be reached by the pipette. Once set up, the microinjection robot can execute 15 or more microinjections per minute. The microinjection robot because of its throughput and versality will make microinjection a broadly straightforward high-performance cell manipulation technique to be used in bioengineering, biotechnology, and biophysics for performing single-cell analyses in organotypic brain slices.
Introduction
This protocol describes the use of a robot to target and manipulate single cells in brain tissue slices, focusing in particular on single neural stem cells and neurons. The robot was developed to address a central question in developmental neurobiology, that is how neural stem and progenitor cells contribute to the brain morphogenesis1,2,3,4,5. To answer this question, one needs to label and track single neural stem cells and follow their lineage progression over time to correlate single cell behavior with tissue morphogenesis. This can be achieved in different ways, e.g., by electroporating brain tissue in utero or by labeling single cell using lipophilic dies. Although powerful, these methods lack precise single cell resolution (electroporation) and/or the possibility to manipulate the intracellular space (lipophilic dye). Microinjection into single cells was developed to overcome this challenge6,7,8. During microinjection, a pipette is briefly inserted into a single cell within intact tissue under pressure to microinject femtoliter volumes of reagents9. We have previously described a manual procedure for microinjecting single neural stem cells in organotypic tissue (Figure 1A)10,11. Microinjection into neural stem cells relies on the use of a micropipette that is inserted into single neural stem cells to inject a solution containing a fluorescent dye, along with other molecules of interest. The selective targeting of neural stem cells is achieved by approaching the developing telencephalon via the ventricular surface (or ventricle, see cartoon in Figure 1A), that is formed by the apical plasma membrane of apical progenitors (cartoon in Figure 1A). This process must be repeated for each cell that the experimenter desires to inject. Further, the success of microinjection is dependent on the precise control of the depth and duration of micropipette injection in the tissue. Thus, despite the unique advantages, manual microinjection is extremely tedious and requires considerable practice to perform at reasonable throughput and yield, making this technique difficult to use in a scalable fashion. To overcome this limitation, we have recently developed an image guided robot, the Autoinjector12 (or microinjection robot) that can automatically perform microinjections into single cells.
The microinjection robot makes use of microscopic imaging and computer vision algorithms to precisely target specific locations in 3-D space within tissue for microinjection (Figure 1B). The microinjection robot can be constructed by making relatively simple modifications to an existing microinjection setup. The overall schematic of the microinjection robot is shown in Figure 1C. A pipette is mounted in a pipette holder attached to a three-axis manipulator. A microscope camera is used to acquire images of the tissue and the microinjection needle. A custom pressure regulation system is used to control the pressure inside the pipette and a programmable micromanipulator is used to control the position of the microinjector pipette. The camera images of the tissue and microinjection pipette are used to determine the spatial location of the microinjection pipette tip and the locations at which microinjections need to be performed. The software then calculates trajectories needed to move the pipette within the tissue. All the hardware is controlled by the software that we previously developed. All software is written in coding language (e.g., Python and Arduino) and can be download from https://github.com/bsbrl/Autoinjector with instructions. The graphical user interface (GUI) allows the user to image the tissue and micropipette, and to customize the trajectory of microinjection. Our system can be established using relatively simple modifications to an inverted microscope equipped with brightfield and epi-fluorescence filters.
First, we provide instructions on preparing brain organotypic tissue slices for microinjection. Then the protocol illustrates starting the microinjection robot followed by preparatory steps, such as pipette motion calibration, that need to be done prior to microinjection. This is followed by defining the injection parameters. After this, the user can define the trajectory used by the microinjection robot and start the injection procedure. The microinjected tissue (in this case brain organotypic tissue slices) can be kept in culture for different time periods depending on the experimental design10,11. The tissue can be processed to follow and study the identity and fate of the injected cells and their progeny. Alternatively, the microinjected cells can be followed using live imaging. Within the scope of this protocol, we demonstrate the use of the robot to automatically microinjection neural progenitor cells in organotypic slices of mouse E14.5 dorsal telencephalon. The robot is further capable of microinjection into newborns neurons in the mouse telencephalon, as well as in the human fetal telencephalon12.
In summary, we describe a robotic platform that can be used to follow and manipulate single cells in tissue. The platform makes use of pressure and it is, therefore, extremely versatile as to the chemical nature of the compound to inject. In addition, it can be adapted to target cells other than stem cells. We expect our system to be easily adapted to other model systems as well.
Protocol
All animal studies were conducted in accordance with German animal welfare legislation, and the necessary licenses were obtained from the regional Ethical Commission for Animal Experimentation of Dresden, Germany (Tierversuchskommission, Landesdirektion Dresden). Organotypic slices were prepared from E14.5 or E16.5 C57BL/6 mouse embryonic telencephalon (Janvier Labs).
1. Installation of software
- Follow instructions to install the software from https://github.com/bsbrl/Autoinjector.
2. Preparation of reagents and pipettes
- Agarose: Prepare 3% agarose by separately dissolving 3 g of wide range agarose and 3 g low-melting point agarose in 100 mL of cell culture-grade PBS in two separate 200 mL glass bottles, respectively. Store at room temperature for up to 3 months.
- Tyrode solution: Dissolve 1 g of sodium bicarbonate and Tyrode’s salt (use the content of the entire bottle) and 13 mL of 1 M HEPES in 1 L of distilled water. Adjust the pH to 7.4. Filter the solution through a 0.2 µm bottle-top filter.
- Slice culture medium (SCM): Add 10 mL of rat serum, 1 mL of 2 mM glutamine, 1 mL penicillin-streptomycin (100x), 1 mL of N-2 supplement (100x), 2 mL of B27 supplement (50x) and 1 mL of HEPES (pH 7.3) buffer into 84 mL of Neurobasal medium. Aliquot 5 mL of SCM into 15 mL tubes. Store at -20 °C.
- CO2-Independent Microinjection Medium (CIMM): Prepare 5x DMEM modified low-glucose solution (without phenol red) by dissolving the powder in 200 mL of distilled water. Filter solution through a 0.2 µm bottle-top filter (for the DMEM powder, use the content of the entire bottle). To prepare 100 mL of CIMM, mix 20 mL of 5x DMEM modified solution, 1 mL of HEPES buffer, 1 mL of N2 supplement (100x), 2 mL of B27 supplement (50x), 1 mL of penicillin-streptomycin (100x), 1 mL of 2 mM glutamine and 74 mL of distilled water. Store the solution at 4 °C.
- Reconstitution buffer: Prepare the reconstitution buffer by dissolving 262 mM NaHCO3, 0.05 N NaOH, 200 mM HEPES in distilled water. Sterilize the solution by filtration through a bottle-top 0.22 µm filter system into a sterile glass bottle. Aliquot 500 µL of reconstitution buffer into airtight microcentrifuge tubes. Store at 4 °C.
- Microinjection dye stock: Dissolve the fluorescently labeled Dextran in RNase free distilled water (final concentration 10 µg/µL). Prepare 5 µL aliquots and store at -20 °C until use.
- Pull the microinjection pipettes from borosilicate glass capillaries (1.2 mm outer diameter, 0.94 mm inner diameter) using the micropipette puller. Protect the pipettes from dust. Do not store pipettes for more than 2 – 3 days. For this experiment, the pulling parameters were HEAT: ramp temperature +1 – 5; PULL: 100; VEL: 110; DEL: 100. HEAT and VEL are the parameters that affect the most shape and size of the pipette.
NOTE: The optimal microinjection pipette has a long and flexible tip, to avoid cell damage during microinjection.
3. Tissue slice preparation
- Melt the 3% wide range agarose using a microwave oven prior to the brain tissue dissection. Do not let the agarose solidify by keeping in a water bath at 37 °C prior to embedding. Ensure that the pipettes are protected from dust. Do not store pipettes for more than 2 – 3 days.
- Thaw an aliquot of SCM and warm 10 – 12 mL CIMM and 20 mL of Tyrode’s solution to 37 °C using a water bath.
- Mix the fluorescent tracer (Dextran-3000 or Dextran-10000-Alexa conjugated; final concentration 5 – 10 μg/μL) with the other chemical(s) to be injected. Centrifuge the microinjection solution at 16, 000 x g for 30 min at 4 °C. Collect the supernatant and transfer into a new tube. Keep the microinjection solution on ice until use.
- Use the heads from E13.5 – E16.5 mouse embryos to prepare organotypic tissue slices of the telencephalon. Remove the skin and open the skull using the forceps, moving along the midline. Dissect out the embryonic brain from the open skull and remove the meninges covering the brain tissue starting from the ventral side of the brain. Leave the dissected whole brain in Tyrode’s solution on a 37 °C heating block.
NOTE: All dissection steps described in 3.4 must be performed in prewarmed Tyrode’s solution. - Pour the wide range melted agarose into a disposable embedding mold. When the agarose is cooled to 38 – 39 °C, carefully transfer the brains (a maximum of 4) into it using a Pasteur pipette. Always use cut tips for this step.
- Stir the agarose around the tissue either using a spatula or a pair of Dumont #1 forceps without touching the tissue. Let the agarose solidify at room temperature. Once the agarose has solidified, trim the excess agarose surrounding the tissue.
- Fill the buffer tray with PBS. Orient the brain with the rostro-caudal axis of the tissue perpendicular to the tray (use as landmark the olfactory bulbs, representing the rostral-most part of the brain). Cut 250 µm slices using a vibratome.
- Fill a 3.5 cm Petri dish with 2 mL of prewarmed media. Using a plastic Pasteur pipette, transfer slices (10 - 15) to this dish. Once done, shift the Petri dish with the slices into the slice culture incubator. Maintain slices at 37 °C in a humidified atmosphere containing 40% O2 / 5% CO2 / 55% N2 until use.
4. Microinjection
- Turn on the computer, microscope, microscope camera, manipulators, pressure rig, and pressure sensor. Load the application by clicking the file “launchapp.py” in the main folder downloaded from GitHub and specify the device settings in the popup screen (see step 1.1 for install instructions).
- Create an outward pressure to prevent unwanted clogging before submerging the pipette into the solution. To apply pressure to the pipette, slide the compensation pressure bar to 24 – 45% and click Set Values. Next, tune the pressure to a sufficient pressure by turning the mechanical pressure valve knob to 1 – 2 PSI (69 – 138 mbar) as indicated by the pressure sensor.
- Transfer the slices to a 3.5 cm Petri dish containing 2 mL of pre-warmed CIMM. Place the slices to be microinjected in the centre of the Petri dish. Transfer the Petri dish to the preheated (37 °C) microinjection stage.
- Load the microinjection pipette with 1.4 –1.6 µL of microinjected solution (from step 3.3) using a long-tip plastic pipette. Insert the microinjection pipette onto the pipette holder.
- Using the lowest magnification on the microscope, bring the slice into focus and guide the micropipette to this field of view (FOV) so that it is focused on the same plane as the slice target. Switch the output of the microscope to the camera to see the FOV in the application.
- Click the magnification button in the top left of the interface to initiate device calibration. A window will prompt to select the magnification. Select the 10x magnification, or whatever magnification the lens is set to (e.g., 4x, 10x, 20x, 40x) and press Ok. The software assumes the internal objective lens is 10x (the most common objective lens magnification).
- Refocus the pipette tip using the micrometric wheel of the microscope and click the pipette tip with the cursor. Next, press the step 1.1 button and press OK in the popup window. The pipette will move in the Y direction. Click the tip of the pipette and press the step 1.2 button. Lastly, enter 45 into the Pipette angle box and press the Set angle.
- Enter desired parameters into the Automated microinjection controls panel. For microinjection into apical progenitors set the injection distance to 20 – 40 µm and depth to 10 – 15 µm. For microinjection into neurons set the injection distance 30 – 40 µm from the basal side, and depth to 10 – 30 µm depending upon what is being targeted. Always set speed to 100%. Click Set values.
NOTE: The approach distance is the distance the pipette pulls out of the tissue before moving to the next injection distance, depth is the depth into tissue the microinjection goes, spacing is the distance along the line between sequential injections, speed is the speed of the pipette in µm/s. - Click the Draw edge button and drag the cursor along the desired trajectory in the popup window to define the trajectory of injection. For microinjecting progenitor stem cells, the ventral side of the telencephalon surface is targeted as shown in Figure 2A. Bring the pipette to the start of the line and click the tip of the pipette. Click Run trajectory to start microinjecting. Repeat this step for every plane of injection targeted (usually done for 3 – 4 planes with 40 – 75 injections per plane).
5. Tissue culture and tissue slice processing for immunofluorescence
- Prepare the collagen mixture (1.5 mg/mL): To a tube add 1.25 mL of the matrix solution, 0.5 mL of distilled water, 0.5 mL of 5x DMEM-F12 solution and 0.25 mL of reconstitution buffer. Keep it on ice until use.
- Obtain the Petri dish containing the microinjected slices from the slice culture incubation chamber and immerse the slices into the collagen mixture.
- Transfer the slices together with 200 – 300 µL of collagen mixture into a 14 mm well of a 35 mm glass-bottom dish. Ensure that the slices are covered in very less collagen. This set up allows for the optimal conditions for nutrients and oxygen uptake.
- Orient the slices while ensuring that there is enough space between the slices using two pairs of forceps. Incubate the Petri dish for 5 min at 37 °C using a heating block to allow for the collagen to solidify. Consider this time as t = 0 of slice culture.
- Move the Petri dish back to the slice culture incubator for an additional 40 min. Then add 2 mL of the prewarmed SCM. Slices are kept in culture until the desired time point.
- Take the slices out of the slice culture incubator and aspirate the SCM. Wash the collagen-embedded slices with 1x PBS. Add 4% (wt/vol) paraformaldehyde (in 120 mM phosphate buffer, pH 7.4) and leave the tissue at RT for 30 min. Then move it to 4 °C to allow for overnight fixation.
- Aspirate the paraformaldehyde solution the next day and perform 1x PBS washes. To remove the slices from the collagen, use two pairs of forceps to gently extract the slices under a stereomicroscope.
- Use a microwave to melt the 3% (wt/vol) low melting point agarose for processing the microinjected slices. Pour the melted agarose into a disposable embedding mold and let it cool to around 38 – 39 °C.
- Transfer the tissue slices from step 5.7 into this mold containing low melting agarose using a plastic Pasteur pipette. Ensure that the pial side of the slice is up and the ventricular surface faces down. If needed orient accordingly. Let the agarose cool down to RT to solidify.
- Trim the extra agarose surrounding the slices. Orient the agarose block to ensure that the cut surface is parallel to the vibratome’s cutting blade. Using vibratome, cut 50 µm thick sections.
- Fill a 24 well dish with 1x PBS. Transfer the sections into this dish using a fine-tip paintbrush. Perform immunofluorescence as per the standard protocols.
Results
Microinjection serves the purpose of tracking and manipulating single neural stem cells and their progeny in living tissue and to follow their lineage progression in a physiological environment. In this article, we have demonstrated the use of the microinjection robot for targeting and automatically microinjecting organotypic slices of the mouse telecephalon. Figure 2 illustrates representative images of successfully injected progenitor cells and Figure 3 illustrates injected newborn neurons. When injected with Dextran Alexa-488 (or Alexa-A555) dye, cells appear fully filled with the dye. As for apical progenitors (Figure 2) confocal imaging allows reconstructing with high spatial resolution the cell morphology, the presence -or absence- of the apical and basal attachment, and to combine the morphological enquiry with marker expression. By combining these criteria, the user can assign a specific cell fate to the microinjected cells and their progeny. As for the neuron injection, the user can reconstruct the neuronal morphology, including the structure and features of apical dendrite and axon. Automated microinjection can provide significantly higher throughput as compared to manual microinjection (Figure 2B). Further, EdU labeling confirms that cell viability is not affected by automation (Figure 2C). Keeping the organotypic slice in culture allows following lineage progression of the microinjected cells (we shown 4 - 24h in Figure 2D). If the microinjection solution contains genetic material (DNA, mRNA, CRISPR-Cas9 guides) or recombinant proteins, then this allows studying if and how lineage progression is affected by the manipulation.
Microinjection into single neural stem cells in tissue provides excellent single cell resolution and for that reason it has been used to dissect the cell biology of neural stem cell progression and fate transition (Figure 3A). Microinjection allows the delivery of complex mixture of chemicals. We previously made use of this feature to study junctional coupling in neural progenitor cells by mixing gap-junctional permeable with gap junctional impermeable fluorescent dyes12. We extended previous work by studying junctional coupling in newborn neurons, by injecting Lucifer Yellow along with Dextran-A555 (Figure 3B). As shown in Figure 3B, a proportion of newborn pyramidal neurons are coupled via gap junctions to neighboring neurons. This observation is consistent with the idea that immature neurons communicate via gap-junction13,14. Furthermore, targeting neurons shows that the use of the microinjection robot can be generalized to several cell types in the developing mammalian brain. This experimental setup will be useful to dissect the cell biology of neurons in tissue, for example by delivering specific oligopeptides to interfere with protein-protein interactions.
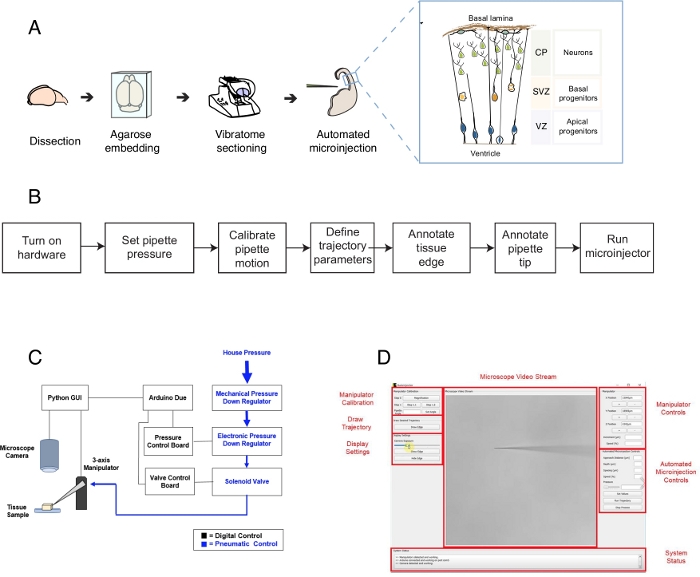
Figure 1: Automated microinjection setup and protocol. (A) Overall protocol for tissue preparation and automated microinjections using the microinjection robot. Right inset: Cartoon schematic of mouse Telencephalon targeted for microinjection in this protocol. (B) Flowchart of the automated microinjection steps. (C) Schematic of the microinjection robot hardware. (D) Graphic user interface (GUI) of the software used to control and operate the microinjection robot. This figure is adapted from ref.12. Please click here to view a larger version of this figure.
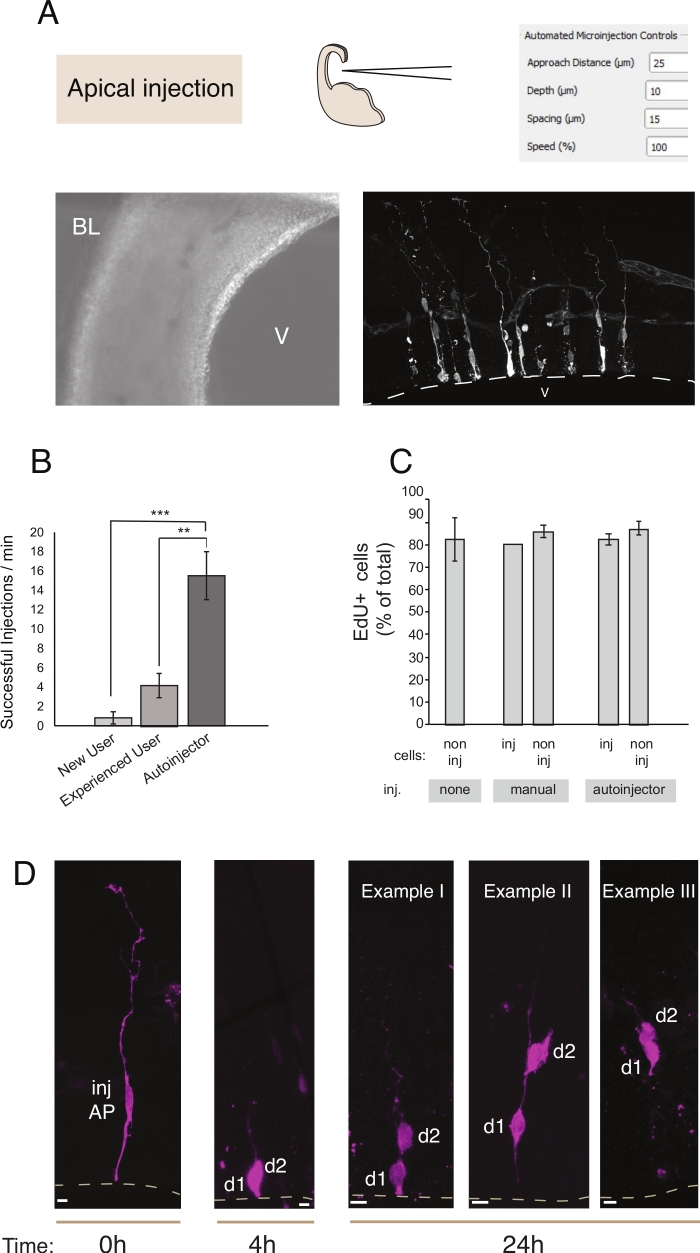
Figure 2: Robotic microinjection into apical progenitors. Schematic and expected results when using the microinjection robot to target apical progenitors (APs) via the apical surface (apical injection). (A) Top row. On the left: schematic of the process. On the right: GUI with relevant parameters for apical injection. Bottom row. On the left: phase contrast image taken during the injection procedure (V: ventricle; BL: basal lamina). On the right: representative results showing microinjected APs. Dashed line represents the ventricle (V). Scale bar: 10 µm. (B) Successful injections per minute for a novice user on the manual microinjection system, an experienced user on the manual microinjection system, and the microinjection robot. (C) EdU incorporation in microinjected cells and in non-injected cells in the injected area. Organotypic slices of mouse E14.5 dorsal telencephalon were either (i) non injected or (ii) subjected to manual or automated microinjection (injected slice) using Dextran-A488 (for manual and autoinjector). Slices were kept in culture in the presence of EdU for 24 h, then they were fixed and stained for DAPI and EdU. Injected and non-injected cells in the injected area were scored for EdU positivity. (D) Use of the microinjection robot Lineage tracing. A fluorescent dye (Dx3-A555, magenta) is injected into single neural stem cell (t = 0 h). The fluorescent dye is partitioned to the daughter cells (d1, d2) during mitosis. This allows following the progeny of the injected cell (t = 4 h and 24 h) and revealing the lineage progression over time. For t = 24 h, we show several examples of the progeny one expects to find. Scale bars: 10 µm. Graphs in B and C are taken from ref.12 Please click here to view a larger version of this figure.
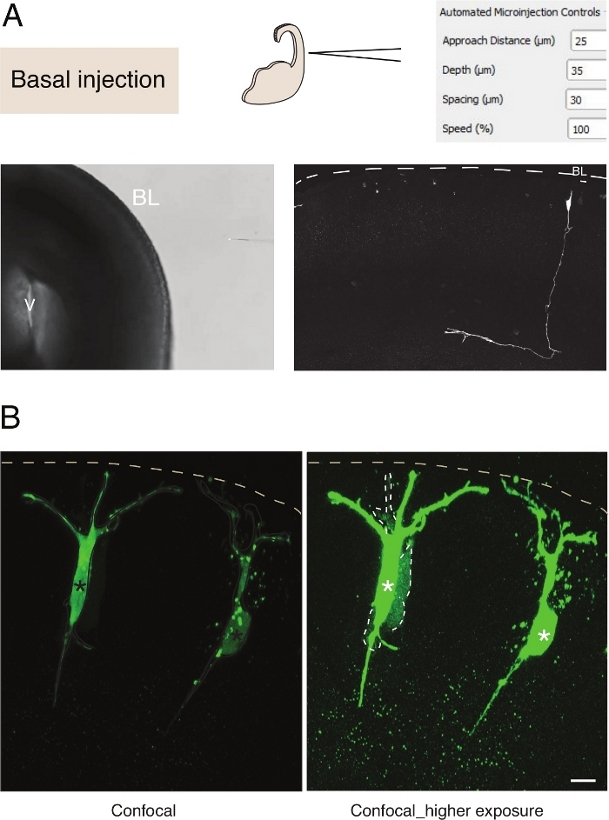
Figure 3: Robotic microinjection into neurons. Schematic and expected results when using the microinjection robot to target pyramidal neurons (N) via the basal surface (basal injection). (A) Top row. On the left: schematic of the process. On the right: GUI with relevant parameters for basal injection. Bottom row. On the left: phase contrast image taken during the injection procedure (V: ventricle; BL: basal lamina). On the right: representative results showing a microinjected N. Dashed line represents the basal lamina (BL). Scale bar: 10 µm. (B) Use of the autoinjector to study gap junctional communication in tissue. Pyramidal neurons were injected with a solution containing two dyes: the gap junctional-impermeable Dx-A555 (not shown) and the gap-junctional permeable Lucifer Yellow (green). Dx-A555 is confined to the targeted cell (asterisks), while the LY diffuses to cells that are connected via gap junction to the targeted cell (dashed lines). Left panel: Low exposure, only the microinjected cells are visible. Right panel: High exposure allows visualization of the injected cells as well as the coupled cells (dashed lines). Scale bar: 10 µm. Please click here to view a larger version of this figure.
Supplementary File: Troubleshooting several common errors that arise during microinjection. Please click here to download this file.
Discussion
Microinjection into single neural stem cells in tissue provides excellent single cell resolution and for that reason it has been used to dissect the cell biology of neural stem cell progression and fate transition (Figure 2; see also10,11,12). The automated microinjection procedure can be performed on other types of cells in both embryonic mice and human brain tissue. Representative results of microinjection of newborn neurons by targeting the basal surface of the telencephalon are shown in Figure 3.
The principle established here can be applied to target several different cell types in embryonic mouse brains and human brains. We have previously shown that the microinjection robot can also be used to target single progenitor cells in the mouse hindbrain and telencephalon and newborn neurons in the mouse and human developing neocortex12. To obtain the best results of the injection procedure, one should optimize all the steps before starting the injection. It is important to carefully consider and optimize the preparation of viable and well preserved organotypic tissue slices from brain tissue (Figure 1). It is crucial to be quick in the dissection and slicing procedure illustrated in Figure 1. For apical injection targeting the APs, one should pick the slices showing the ideal orientation of the apical surface. For APs injection, the ideal orientation is the apical surface perpendicular to the bottom of the Petri dish. Any other orientation will be permissive as well, however, the apical surface perpendicular to the Petri dish provides a wider surface area for injection, thus increasing the success of injection. For injection into neurons, the orientation of the slice plays little to no effect.
Once the slices to inject are selected, the injection procedure per slice takes approximately 5 minutes. Considering that one works with living tissue, it is highly recommended to speed up the injection procedure. To this end we recommend setting all the parameters for injection via the GUI (Figure 1D) before the tissue is ready, to reduce any unnecessary waiting time. For troubleshooting please refer to the Supplementary file.
In case of long-term slice culture, steps after the automated microinjection procedure can affect the health of the cells and thereby the experiment. Therefore, it is highly recommended to run a quality control test and to optimize the slice culture conditions. To evaluate cell viability after the slicing and injection procedure, we performed EdU labeling during the culture and we quantified the number of pyknotic nuclei (a proxy for apoptotic cells) in the cultures and injected tissue12. These quantifications did not reveal any significant impact of microinjection on tissue viability (Figure 2C). We recommend running similar quality controls while establishing the organotypic tissue slicing and microinjection pipeline in the lab.
Compared to manual microinjection, the microinjection robot provides several advantages. First, the learning curve for the user is less steep as compared to manual injection: a new user will reach a high proficiency after a limited number of sessions, typically 1 or 2. Second, in the case of manual microinjection, a comparable proficiency requires months of training. The injection procedure is faster and more efficient (Figure 2B). We quantified these parameters and found that the microinjection robot outperformed a skilled manual user with respect to the injection success (% of successful injection/total number of injections) and in the total number of injections per unit time12. This results in an overall 300% increase of injection efficiency (% of successful injection/min) for the microinjection robot compared to a skilled user. The increase in efficiency was even more pronounced when comparing the microinjection robot with a beginner user and reached 700%. Last but not the least, the microinjection robot can be easily programmed to systematically explore all spatial parameters. This is particularly advantageous when adapting the microinjection robot to target new cells or tissues, or when using the microinjection robot for purposes requiring different spatial resolution.
Building the microinjection robot requires minimal changes to an existing epi-fluorescence microscope12. We have previously provided instructions for this adaptation at https://github.com/bsbrl/Autoinjector. Once the hardware is setup, this protocol provides key methodological details for successfully undertaking automated microinjections. Overall, the microinjection robot has a successful injection rate of 15.52 + 2.48 injections/min, which is 15x greater than an inexperienced user (1.09 ± 0.67 injections/min), and 3x greater than an expert user (4.95 ± 1.05 injections/min)12. This improvement in successful injection rate empowers both novice and expert users to inject more cells in a shorter amount of time which is essential to preserve tissue viability. Additionally, the microinjection robot is customizable and the trajectory, depth of the injection, number of injections, spacing between injections can all be tuned using the GUI. These features allow the microinjection robot to be used as a tool to optimize previously laborious experiments, and to explore fundamentally new experiments that require higher yield than previously possible.
The main limitations of the microinjection procedure we described here are related to the preparation of tissue slices, a crucial step that needs extensive optimization. In addition, microinjection relies on the presence of a surface that can be approached by the glass pipette. This feature limits the type of tissues and tissue locations that can be targeted via microinjection using the present setup.
The microinjection robot currently uses brightfield imaging and has been used in in vitro brain slice preparations. In the future, the microinjection robot could be combined with 2-photon imaging to increase the specificity of single cell targeting in vivo for molecular or dye tagging. Such efforts have already been made for single cell electrophysiology15,16. The current device requires manual observation of the microinjection procedure. Future versions could include strategies for cleaning clogged microinjection pipettes17 or integration of fluid handling robots18 for multiplexed, fully autonomous microinjections. These devices could increase the scale of microinjection by orders of magnitude. Adapting algorithms for parallel control of multiple microinjection pipettes19 could enable multiplexed delivery of dozens of dyes and molecular reagents into the same cells within the same experiments. This has the potential to open new avenues for molecular screening in tissue.
The microinjection robot could be used to tag functionally identified cells using DNA or RNA barcodes. This could in turn be combined with other single cell analysis techniques, such as single cell RNA sequencing (scRNAseq) and electron microscopy. Our preliminary results show that microinjected cells and their progeny can be recovered and isolated using tissue dissociation followed by FACS sorting (Taverna, unpublished results). The FACS sorted cells can then be used for scRNAseq. Furthermore, preliminary results show that the single cell resolution capabilities of the microinjection robot can be used in combination with electron microscopic analysis to explore the cell biology on neural stem cells in tissue at high spatial resolution (Taverna and Wilsch-Bräuninger, unpublished results). These data suggest that the microinjection robot can be used as a tool for correlative light and electron microscopy in tissue and in broader sense, for the multimodal analysis of cell identity and behavior in tissue.
Microinjection relies on the use of pressure and one can afford injecting solutions with high molecular complexity (e.g., an entire transcriptome). This feature of microinjection has been exploited in the past for isolating and cloning ligand-gated receptors20. Along this line, the microinjection robot might be used for modeling and studying multi-genic traits at the cellular level. Combined with a sub-pooling strategy, the microinjection robot might also be used as a platform to identify the minimum set of genes driving a certain trait/cellular behavior. Thus far, the microinjection robot has been used to manipulate the cell’s biochemistry via the delivery of mRNA, DNA or recombinant proteins10,21,22. We foresee an application of the microinjection robot in probing the biophysics of the intracellular space, for example, by delivering nanomaterials or nanomachines that allow sensing and/or manipulation of the biophysical properties of the intracellular space.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the Nomis Foundation (ET). SBK acknowledges funds from the Mechanical Engineering department, College of Science and Engineering, MnDRIVE RSAM initiative of the University of Minnesota, Minnesota department of higher education, National Institutes of Health (NIH) 1R21NS103098-01, 1R01NS111028, 1R34NS111654, 1R21NS112886 and 1R21 NS111196. GS was supported by National Science Foundation Graduate Research Fellowship and NSF IGERT training grant.
Materials
| Name | Company | Catalog Number | Comments |
| Chemicals | |||
| Agarose, Low Melt | Carl Roth | Cat# 6351.2 | |
| Agarose, Wild Range | Sigma-Aldrich | Cat# A2790 | |
| Best-CA 221 Glue | Best Klebstoffe GmbH & Co.KG | Cat# CA221-10ml | |
| B-27 Supplement | Thermo Fisher Scientific | Cat# 17504044 | |
| Cellmatrix Type-IA (Collagen, Type !) | FUJIFILM Wako Chemicals | Cat# 637-00653 | |
| Distilled Water | |||
| DMEM-F12, CO2 independent (w/o Phenol red) | Sigma-Aldrich | Cat# D2906 | |
| DMEM-F12, CO2 independent (with Phenol red) | Sigma-Aldrich | Cat# D8900 | |
| HEPES-NAOH, pH 7.2, 1M (HEPES buffer) | Carl Roth | Cat# 9105.3 | |
| L-Glutamine, 200 mM | Thermo Fisher Sientific | Cat# 25030024 | |
| Mowiol 4-88 | Sigma-Aldrich | Cat# 81381 | |
| N-2 Supplement | Thermo Fisher Scientific | Cat# 17502048 | |
| Neurobasal Medium | Thermo Fisher Scientific | Cat# 21103049 | |
| Nuclease-free water | Thermo Fisher Scientific | Cat# AM9937 | |
| O2 (40%), CO2 (5%), N2 (55%) Mix, 50 liters | |||
| Paraformaldehyde | Merck | Cat# 818715 | |
| PBS | |||
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | Cat# 15140122 | |
| Rat serum | Charles River Laboratories | ||
| Japan | |||
| Sodium bicarbonate (NaHCO3) | Merck | Cat# 106323 | |
| Sodium hydroxide (NaOH) | Merck | Cat# 106482 | |
| Tyrode’s salt | Sigma | Cat# T2145-10x1L) | |
| Equipment | |||
| Borosilicate glass capillaries, 1.2 mm outer diameter x 0.94 mm inner diameter | Sutter Instruments | Cat# BF-120-94-10 | |
| Bottle-top filter system, 500 mL | Corning | Cat# 430769 | |
| Computer PC | |||
| Custom pressure rig | Custom pressure rig | ||
| Electronic pressure regulator | Parker Hannifin | Cat# 990-005101-002 | |
| Falcon tubes, 15 mL | Corning | Cat# 430791 | |
| Falcon tubes, 50 mL | Corning | Cat# 430829 | |
| Fine-tip paintbrush | |||
| Flaming/ Brown micropipette puller | Sutter Instruments | Cat# P-97 | |
| Forceps, Dumont no. 3 | Fine Science Tools | Cat# 11231-30 | |
| Forceps, Dumont no. 5 | Fine Science Tools | Cat# 11255-20 | |
| Forceps, Dumont no. 55 | Fine Science Tools | Cat# 11252-20 | |
| Heating block | Labtech International | Cat # Dri block Digi2 | |
| Inverted fluorescence microscope | Zeiss | Cat# Axiovert 200 | |
| Light source | Olympus | Cat# Highlight 3100 | |
| Manual pressure regulator | McMaster Carr | Cat# 0-60 PSI 41795K3 | |
| Microloader Tips | Eppendorf | Cat# 5242956.003 | |
| Microcontroller | Arduino | Cat# Arduino Due | |
| Microscope camera Hamamatsu Orca Flash 4.0 V3 | |||
| Motorized stage XY for microscope | |||
| Multiwell plate, 24 wells | Nunc | Cat# 142475 | |
| Pasteur pipettes, plastic | |||
| Petri dish, 60 x 15 mm | Greiner | Cat# 628102 | |
| Petri dish, 35 x 10 mm | Nunc | Cat# 153066 | |
| Petri dish, 34 x 14 mm, including Microwell no. 1.5 cover glass | MatTek | Cat# P35G-1.5-14-C | |
| Pipette holder | Warner Instruments | Cat# 64-2354 MP-s12u | |
| Pipette and tips | |||
| Puller filament, 3.0-mm square box filament | Sutter Instrument | Cat# FB330B | |
| Slice culture incubation box | MPI-CBG | Cat# custom made | |
| Solenoid valve | Cat# LHDA053321H-A | ||
| Stereomicroscope | Olympus | Cat# SZX12 | |
| Tabletop centrifuge | Heraeus | Cat# 5431622 | |
| Thermometer | |||
| Three-axis Manipulator | Sensapex Inc | Cat# tree-axis uMP | |
| Vibratome | Leica | Cat# VT1000s | |
| Whole-embryo-culture-system incubator | Ikemoto Company | Cat# RKI-10-0310 | |
| Waterbath | |||
| Software and Algorithms | |||
| Arduino | Arduino | ||
| Fiji | RRID: SCR_002285 | ||
| Python | Python Software foundation | Python 2.7.12 | |
| ZEN | RRID: SCR_013672 |
References
- Taverna, E., Götz, M., Huttner, W. B. The Cell Biology of Neurogenesis: Toward an Understanding of the Development and Evolution of the Neocortex. Annual Review of Cell and Developmental Biology. 30 (1), 465-502 (2014).
- Götz, M., Huttner, W. B. The cell biology of neurogenesis. Nature Reviews Molecular Cell Biology. 6 (10), 777-788 (2005).
- Di Lullo, E., Kriegstein, A. R. The use of brain organoids to investigate neural development and disease. Nature Reviews Neuroscience. 18 (10), 573-584 (2017).
- Lancaster, M. A., Knoblich, J. A. Organogenesisin a dish: Modeling development and disease using organoid technologies. Science. 345 (6194), 1247125 (2014).
- Kretzschmar, K., Clevers, H. Organoids: Modeling Development and the Stem Cell Niche in a Dish. Developmental Cell. 38 (6), 590-600 (2016).
- Pepperkok, R. et al. Automatic microinjection system facilitates detection of growth inhibitory mRNA. Proceedings of the National Academy of Sciences of the United States of America. 85 (18), 6748-6752 (1988).
- Pepperkok, R., Lowe, M., Burke, B., Kreis, T. E. Three distinct steps in transport of vesicular stomatitis virus glycoprotein from the ER to the cell surface in vivo with differential sensitivities to GTPγS. Journal of Cell Science. 111 (13), 1877-1888 (1998).
- Pepperkok, R. et al. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 74 (1), 71-82 (1993).
- Ansorge, W., Pepperkok, R. Performance of an automated system for capillary microinjection into living cells. Journal of Biochemical and Biophysical Methods. 16 (4), 283-292 (1988).
- Taverna, E., Haffner, C., Pepperkok, R., Huttner, W. B. A new approach to manipulate the fate of single neural stem cells in tissue. Nature Neuroscience. 15 (2), 329-337 (2012).
- Wong, F. K., Haffner, C., Huttner, W. B., Taverna, E. Microinjection of membrane-impermeable molecules into single neural stem cells in brain tissue. Nature Protocols. 9 (5), 1170-1182 (2014).
- Shull, G., Haffner, C., Huttner, W. B., Kodandaramaiah, S. B., Taverna, E. Robotic platform for microinjection into single cells in brain tissue. EMBO Reports. 20 (10), e47880 (2019).
- Jabeen, S., Thirumalai, V. The interplay between electrical and chemical synaptogenesis. Journal of Neurophysiology. 120 (4), 1914-1922 (2018).
- Nagy, J. I., Pereda, A. E., Rash, J. E. Electrical synapses in mammalian CNS: Past eras, present focus and future directions. Biochimica et Biophysica Acta - Biomembranes. 1860 (1), 102-123 (2018).
- Suk, H.J. et al. Closed-loop real-time imaging enables fully automated cell-targeted patch-clamp neural recording in vivo. Neuron. 95 (5), 1037-1047 (2017).
- Annecchino, L. A. et al. Robotic automation of in vivo two-photon targeted whole-cell patch-clamp electrophysiology. Neuron. 95 (5), 1048-1055 (2017).
- Kolb, I. et al. Cleaning patch-clamp pipettes for immediate reuse. Scientific Reports. 6, 35001 (2016).
- Holst, G. L. et al. Autonomous patch-clamp robot for functional characterization of neurons in vivo: development and application to mouse visual cortex. Journal of Neurophysiology. 121 (6), 2341-2357 (2019).
- Kodandaramaiah, S. B. et al. Multi-neuron intracellular recording 1 in vivo via interacting autopatching 2 robots. ELife. 7, 24656 (2018).
- Lubbert, H. et al. cDNA cloning of a serotonin 5-HT1c receptor by electrophysiological assays of mRNA-injected Xenopus oocytes (RNA fractionation/hybrid depletion/hybrid selection/choroid plexus/voltage clamp). Neurobiology. 84 (2) 4332-4336 (1987).
- Florio, M. et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science. 347 (6229), 1465-1470 (2015).
- Kalebic, N. et al. CRISPR/Cas9-induced disruption of gene expression in mouse embryonic brain and single neural stem cells in vivo. EMBO Reports. 17 (3), 338-348 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved