Method Article
Generation of Greater Bacterial Biofilm Biomass using PCR-Plate Deep Well Microplate Devices
In This Article
Summary
This protocol presents methodology to perform biofilm growth and biomass measurements using self-assembled deep well PCR-plate devices for high-throughput 96-well pegged lid static biofilm screening.
Abstract
Bacterial biofilms are difficult to eradicate from surfaces using conventional antimicrobial interventions. High-throughput 96-well microplate methods are frequently used to cultivate bacterial biofilms for rapid antimicrobial susceptibility testing to calculate minimal biofilm eradication concentration (MBEC) values. Standard biofilm devices consist of polystyrene pegged-lids fitted to 96-well microplates and are ideal for measuring biofilm biomass and MBEC values, but these devices are limited by available peg surface area for biomass accumulation and cost. Here, we outline a protocol to use self-assembled polypropylene 96-well deep well PCR-plate pegged-lid device to grow Escherichia coli BW25113 and Pseudomonas aeruginosa PAO1 biofilms. A comparison of 24-hour biofilms formed on standard and deep well devices by each species using crystal violet biomass staining and MBEC determination assays are described. The larger surface area of deep well devices expectedly increased overall biofilm formation by both species 2-4-fold. P. aeruginosa formed significantly greater biomass/mm2 on deep well pegs as compared to the standard device. E. coli had greater biomass/mm2 on standard polystyrene devices as compared the deep well device. Biofilm eradication assays with disinfectants such as sodium hypochlorite (bleach) or benzalkonium chloride (BZK) showed that both compounds could eliminate E. coli and P. aeruginosa biofilms from both devices but at different MBEC values. BZK biofilm eradication resulted in variable E. coli MBEC values between devices, however, bleach demonstrated reproducible MBEC values for both species and devices. This study provides a high throughput deep well method for growing larger quantities of biofilms on polypropylene devices for downstream studies requiring higher amounts of static biofilm.
Introduction
Pseudomonas aeruginosa and Escherichia coli are Gram-negative proteobacteria that are commonly studied for their ability to form sessile, surface-attached cell communities known as biofilms1. Both species, when growing as biofilms, can secrete a matrix of extracellular polymeric substances (EPS) composed primarily of different polysaccharides and proteins, that can also include extracellular DNA and/or lipids, providing added cellular protection and enhanced survival in harsh, nutrient-limited environments2,3. Biofilm physiology and formation by both species is of clinical relevance, as they represent the top five most commonly isolated antimicrobial resistant pathogens from hospital patient blood, urinary tract and lung infections in Canada4,5. It is also important to note that biofilms are estimated to make up nearly 80% of all chronic and recurrent infections caused by these bacterial species6. Due to the secreted EPS substances and slower metabolic activity7,8, established biofilms become more difficult to eradicate when they form on surfaces such as implanted medical devices, scar tissues, and in the lungs of cystic fibrosis patients9,10, adding to their antimicrobial resistance.
The recalcitrant growth properties of bacterial biofilms often make them more resistant to antimicrobial inhibition and/or eradication2,9. Thus, establishing in vitro methods to study bacterial biofilm antimicrobial eradication is paramount for selecting effective compounds to eradicate infections when they form on medical plastics (e.g., in-dwelling catheters) and medical implants. Most rapid in vitro biofilm antimicrobial eradication culturing methods examine biofilm growth as a "static" biofilm culture rather than "continuous" cultures, where static bacterial growth is monitored in early to late stages of biofilm formation over a short (24-96 h) timeframe in the same growth medium. Continuous biofilm methods are cumbersome for rapid analysis as they require biofilm growth assessed over longer timeframes grown in chambers that allow the continuous flow and replacement of growth media with fewer replicates11. Due to the time and effort required to maintain and establish continuous biofilms, static in vitro biofilms are the most popular, as they are easily adapted for high-throughput antimicrobial susceptibility testing assays in plastic 96-well microtiter plates rather than elaborate flow chamber systems that limit the number of cultures simultaneously tested 12,13. The simplest static "in-well" biofilm microplate assays use standard polystyrene or vinyl (300 µL capacity) microtiter plates to measure biofilm formation on the sides and bottoms of each well and often as rings at the air to liquid surface interface. Bacterial in-well biofilm formation is measured by staining the deposited biomass adhered to the wells after the growth medium liquid is removed and the biofilms are washed12,13. These assays are economically popular but often have reproducibility issues due to their inherent design, as deposited biofilms are prone to damage or loss during rinsing for cell recovery and biomass staining procedures11,14.
To reduce biofilm losses, standard commercial biofilm devices have improved upon in-well biofilm microplate designs by adding an insertable 96-well pegged polystyrene lid to the standard 96 in-well plate design, referred to as the "standard biofilm device" herein. The addition of a pegged lid expands the available surface area in each microplate well, allowing for enhanced surface adherence and biofilm biomass formation15,16. Standard biofilm pegged lid devices allow for greater biofilm recovery, removal, and rinsing for subsequent antimicrobial biofilm susceptibility and eradication testing when pegged lids are inserted into new microtiter plates containing drug or growth condition challenges. Similar to in-well biofilm microplate techniques, the recovered materials from the removed and washed pegged lid devices allows for cell survival testing and biofilm biomass staining, typically involving crystal violet (CV) dye formulations 17,18,19. Standard biofilm devices are also optimal for screening biofilm antimicrobial susceptibilities. These assays monitor biofilm growth inhibition in two ways: 1) When antimicrobials are added to cells at the start of growth, it can determine the minimum biofilm inhibition concentration (MBIC) value. 2) When established biofilms are formed on the pegs after 24 hours and then exposed to antimicrobials, it can determine the minimum biofilm eradication concentration (MBEC) value 17,20. Similar to in-well biofilm microplate devices, standard biofilm devices have some notable limitations, such as their high cost per device, they are non-autoclavable, and less durable to chemical solvents due to the polystyrene plate material used. Standard biofilm devices also have a low surface area to peg ratio, which limits the maximum working volumes in each well to 200 µL. These factors can make standard biofilm devices more challenging to use for studies that demand greater quantities of biofilm in a high-throughput format.
Here, we describe a static biofilm pegged-lid 96-well method developed in our lab using commercially available polypropylene semi-skirted 0.5 mL 96-well PCR plates fitted to 96-well microtiter plates having wells deeper than the standard plate (Table of Materials). These assembled devices have a maximum working volume of 750 µL when used for growing biofilm (herein known as "deep well biofilm device"). The advantages of using these deep well biofilm devices are their lower cost when compared to standard biofilm devices, they can be sterilized by autoclaving, and they increase the surface area for biofilm formation on the larger external PCR-plate "tube/pegs". With this method we show the applications of these devices for growing and characterizing biofilm biomass formed by Escherichia coli BW25113 and P. aeruginosa PAO1. Methods to determine MBEC values using biofilm eradication assays are described using the quaternary ammonium compound disinfectant benzalkonium chloride (BZK) and sodium hypochlorite (bleach) antimicrobials. The antiseptic/disinfectant BZK was selected as this compound is frequently used to inhibit biofilms from contaminated surfaces, but it is reportedly less effective at eradicating well-established biofilms21. Bleach is a highly effective chemical for the eradication of established biofilms and is a mainstay in chemical disinfection 22. Both disinfectants provide a useful comparison of MBEC values for each biofilm device21. A protocol for this biofilm device assessment using CV staining and biofilm eradication assays for MBEC determination is summarized in this article. A protocol flowchart has been included for a simplified overview of the workflow for these methods (Figure 1).
Protocol
1. Aseptic culture preparation for biofilm growth (Days 0-1)
- On Day 0, streak the desired bacterial strain(s) for testing from a cryo-preserved stock (in glycerol or dimethylsulfoxide (DMSO)) directly onto a nutrient agar plate with antimicrobial selection as the strain requires. Incubate agar plates at the optimal temperature (37 °C) and time (18-22 h) for strain growth.
- The following day (Day 1), inoculate a colony from the agar plate in 5 mL of growth media at optimal growth conditions. These cultures will be used as Day 2 starting inoculates for biofilm devices (see step 2.2; Figure 1).
NOTE: For this study, E. coli K-12 BW21153 and P. aeruginosa PAO1 were grown in Luria-Bertani (LB) medium at 37 °C for 18 h with shaking at 160 revolutions per minute (rpm).- Prepare at least 3 independently cultured strains to generate biological replicates for biofilm measurement due to the statistical variability of biofilm biomass formation on the pegged lid devices.
2. Preparation and inoculation of plates (Day 2)
- Fill the outer wells of an autoclaved 96-well, 1.1 mL/well polypropylene plate with 750 µL/well sterile water (Figure 2A). Fill the negative control wells (uninoculated media wells; column B) with 750 µL growth media and the remaining wells with 675 µL growth media.
NOTE: Step 2.1 above and steps below list appropriate volumes for the deep well biofilm device. If using the standard biofilm device, the volumes are to be changed as follows: 75 µL of inoculum culture to 20 µL; 675 µL of growth media to 180 µL; 750 µL of sterile water/ growth media to 200 µL; 800 µL of CV stain/acetic acid/ rinse solutions to 210 µL. Additionally, for step 3.6 below, the Absorbance at 500nm (A550 nm) can be read directly in the polystyrene microplate after mixing when using the standard biofilm device microplate. - Using Day 1 overnight cultures, standardize the cultures to an optical density at 600 nm (OD600nm) =1.0 or to a McFarland standard of 0.5-1 units.
- Create a 10-fold serial dilution of each standardized culture in test tubes or a 96-well deep well microtiter plate until a 10-3 dilution is reached.
- Inoculate the media containing wells as shown in Figure 2B with 75 µL of the 1 x 10-3 diluted culture, for a final in-well bacterial dilution of 10-4.
- Carefully insert an autoclaved PCR plate (pegged lid) into the inoculated deep well plate. Incubate plates at optimal strain conditions (37 °C) with shaking (maximum 160 rpm) in an incubator with 50-60% humidity for 24 h.
3. Biofilm biomass determination from devices using CV staining (Day 3)
- Aseptically remove the biofilm pegged lid from each device and rinse by inserting into an autoclaved deep well plate prepared with 800 µL of sterile phosphate buffered saline (PBS)/well for 30 s to remove planktonic cells.
- For biomass CV staining, transfer the pegged PCR plate lid into a new plate prepared with 800 µL/well 0.1% (w/v) CV in dH2O and stain for 5 minutes.
- Remove excess stain by rinsing the pegged lid in a deep well plate prepared with 800 µL/well sterile PBS. Allow the plates to dry for 10 minutes in a biosafety cabinet with pegs facing upward.
- Transfer the dried PCR plate lid to a plate containing 800 µL/well 30% (v/v) acetic acid and allow the lid to de-stain for 5 minutes.
- Remove the destained PCR peg lid and mix the solution thoroughly by pipetting. Transfer 200 µL from the deep well plates to a standard 96 well microplate and measure the absorbance of the de-staining solution at a wavelength of 550 nm (A550nm) in an ultraviolet (UV)/ visible (Vis) range microplate reader.
- Using the measured A550nm values, subtract the average blank (negative control) A550nm value from each biofilm sample value. Average each blank-subtracted A550nm biofilm sample value representing sample biological replicates.
4. Challenging biofilms with antimicrobials to calculate their 24 h antimicrobial MBEC value (Day 3)
- Prepare an antimicrobial stock solution in water that is two times (2x) the concentration of the desired highest antimicrobial concentration to be tested.
- Create a two-fold dilution series of the antimicrobial stock solution in 2x concentrated growth media so that there are 8 antimicrobial concentrations.
- As shown in Figure 2B, fill the outer wells of an autoclaved deep well plate with 750 µL/well sterile water. Fill columns B (negative control; no bacteria) and C (positive control; no antimicrobial exposure) of the plate with 750 µL/well growth media. Fill the remaining wells with 750 µL/well of the antimicrobial dilution series as shown in Figure 2B.
- Remove and rinse the pegged lid as described in step 3.1 and transfer them into the antimicrobial challenge plate.
- Incubate plates with shaking (max 160 rpm) in appropriate strain conditions and for the desired exposure timeframe.
5. Recovery of biofilm biomass from pegged lids (Day 4)
- Remove antimicrobial exposed pegged lids and rinse as described in step 3.1. Transfer the pegged lid to a deep well plate with 750 µL/well recovery media in the inner wells and 750 µL/well sterile dH2O in the outer wells.
- To prepare 70 mL recovery media, combine 35 mL of 2x concentrated LB, 0.7 mL of 100% tween-20, 3.5 mL of 20x universal neutralizing solution, and 30.8 mL of sterile dH2O in a sterile bottle.
- To prepare 20x concentrated universal neutralizing solution, add 1.0 g of L-histidine, 1.0 g of L-cysteine, and 2.0 g of reduced glutathione to a final volume of 20 mL of dH2O. Filter sterilize the solution through a 0.2 µm filter and store at 4 °C.
- Put the device from step 5.1 into a secondary bin fitted inside a sonicating water bath. Sonicate the devices for 30 minutes to dislodge the biofilm from pegs into the recovery media.
- After sonication, remove the pegged lid and place a sterile flat plate cover lid on the deep well recovery media plate. The pegged lid can be discarded.
- Incubate plates with recovery media from step 5.3 at the optimal strain growth conditions overnight (16-18 h).
6. Determination of device MBEC values (Day 5)
- The following day, transfer 200 µL from each well of the incubated deep well plate from step 5.4 to a new 96-well microtiter plate. Read the OD600nm of each well using a UV/Vis range microplate reader.
- Subtract negative control (uninoculated blank) OD600nm values from each biofilm containing sample well.
- Calculate the MBEC value for each sample using OD600nm values, where the MBEC value is the lowest antimicrobial concentration that resulted in the lowest OD600nm value (indistinguishable from the uninoculated control) for a specific antimicrobial treated species/strain sample.
Results
The aim of this study was to provide a method for conducting larger volume, high-throughput (96-well), static biofilm device measurements using deep well biofilm devices. Here, we compared a self-assembled semi-skirted PCR-plate inserted into a deep well microplate, known as the deep well device, to a commonly used standard biofilm pegged lid device to examine their capabilities to form static biofilms. CV-stained biomass and biofilm eradication assays (MBEC) were used to assess biofilm formation by both devices.
To compare biofilm formation by different species on each device, we assessed biofilm biomasses formed by E. coli BW25113 and P. aeruginosa PAO1 using the biofilm CV staining protocol17. Although CV staining is generally reported as A550nm values, due to the differences in growth volumes of each device and their available surface areas we converted CV stain A550nm values to molar CV concentrations in solution. Molar CV concentrations accounted for volume differences of each device and allowed a comparison of CV concentrations representing biomasses recovered from each device. The results showed that both species produced significantly more biomass on deep well biofilm devices (2.1 fold E. coli; 4.1 fold P. aeruginosa) compared to the standard biofilm device (Figure 3A-B). This outcome was expected given the larger surface area of the deep well PCR peg (320.4 mm2) as compared to the standard device peg surface area (108.9 mm2). This finding is also consistent with the increased the increased volumes (750 µL) used in the deep well biofilm device as compared to the standard device wells (200 µL). Hence, deep well devices increased biofilm biomass accumulation on PCR tube pegs as compared to smaller pegs with standard biofilm devices.
Both biofilm devices formed reproducible biofilms when we compared biologically replicated biofilms formed by E. coli and P. aeruginosa (Figure 3A-B). Despite observing greater variability in technical replicate CV-stained M A550nm values for either strain grown on the deep well device, there were no statistically significant differences when we compared the median CV M values for each species' biological replicates on the deep well or standard devices using pairwise two-way Analysis of variance (ANOVA) or Student's T-tests (both p > 0.05). This finding shows that biofilm formation by deep well and standard devices form reproducible biofilms. However, the CV-staining results also showed that when we accounted peg surface area differences on each device, the biofilm biomass formation by E. coli and P. aeruginosa showed statistically significant differences in biomass accumulation (Table 1). Calculation of mean CV-stained biomass (M) per peg surface area in mm2 (CV M/mm2) for E. coli showed that biofilm formation was 1.5-fold lower on polypropylene deep well devices when compared to the standard biofilm device (Table 1). However, the opposite result was obtained for P. aeruginosa, which demonstrated 1.4-fold higher CV M/mm2 on polypropylene deep well devices when compared to standard biofilm devices (Table 1). Despite the observed species-specific differences in biofilm biomass accumulation with each device, the deep well device still demonstrated greater overall (2-4-fold increases) biofilm biomass formation by each species (Figure 3).
To determine the antimicrobial susceptibility testing applications of the deep well biofilm device, we compared two commonly used disinfectants, BZK and bleach, for their biofilm eradicating potential. Both chemicals are commonly used to prevent (BZK) and/or eradicate (bleach) bacterial biofilms in clinical and industrial applications21,22,23. Each compound was added in increasing 2-fold concentrations to biofilms formed by E. coli and P. aeruginosa on the standard and deep well biofilm devices22,23(Figures 1, 2B). The lowest concentration of BZK or bleach that resulted in OD600nm values that were indistinguishable from negative control wells were defined as the MBEC value. Treatment of biofilms formed on standard biofilm devices with bleach resulted in the same bleach MBEC values of 0.625% for E. coli and P. aeruginosa (Table 1, Figure 4A-B). Bleach MBEC values determined for E. coli and P. aeruginosa biofilm formed on deep well devices showed 2-4 fold lower MBEC values by both species (E. coli; 0.156%; P. aeruginosa 0.313%) when compared to standard devices (Table 1). A 2-fold difference in bleach MBEC values between species was noted for the deep well device, where P. aeruginosa required a 2-fold higher concentration of bleach to eradicate biofilms as compared to E. coli (Figure 4A-B, Table 1). The 2-fold bleach MBEC difference between P. aeruginosa and E. coli in deep well device appears to inversely correlate with 1.5-fold increased CV-stained biomass formation by P. aeruginosa as compared to E. coli (Figure 3A-B). The larger amount of biomass formed by P. aeruginosa on deep well device pegs may also explain why a higher bleach concentration was required to eradicate P. aeruginosa biofilms when compared to E. coli on deep wells (Figure 3A-B, Table 1). Hence, deep well device biofilm eradication assays show that both species were susceptible to bleach but at lower (2-4-fold) bleach concentrations as compared to standard devices. This inversely correlates with the 3-fold greater peg surface area and volumes of the deep well device PCR plate pegs. This suggests that for bleach exposure, greater biomass surface area may lower the bleach concentrations necessary for biofilm eradication.
BZK biofilm eradication testing showed greater variability in recovered growth (OD600nm values) and MBEC values by each species when compared to bleach results (Figure 4C-D). This variability is not unexpected based on previous studies showing that BZK was more effective at preventing biofilm formation than eradicating well-established biofilms24,25,26. Using the standard biofilm device, only P. aeruginosa biofilms treated with BZK showed a consistent MBEC value (2560 µg/mL) that was 8-fold lower than the MBEC value (20480 µg/mL) obtained for this strain in the deep well device (Table 1, Figure 4C). These results may reflect differences in the amounts of P. aeruginosa biomass formed on deep well pegged surfaces and plastic compositions when compared to the standard device pegs. BZK eradication of biofilms formed by E. coli on both the deep well and standard devices were equally poor, resulting in a broad range of BZK MBEC values from 2560-40960 µg/mL for the deep well device and 320-10240 µg/mL on the standard device (Figure 4D). For E. coli, this variability was explained by the occasional instance where 1-2 replicate wells showed low but statistically significant growth in recovery media, that increased error and reduced BZK MBEC determination accuracy with either device (Figure 4D, Table 1). This variability highlights the ineffectiveness of using BZK in eradicating E. coli biofilms as previously noted in studies24,25,26. In contrast P. aeruginosa biofilms could be reliably eradicated by BZK at a defined concentration with both devices, but with an 8-fold BZK concentration difference (Figure 4C). In summary, BZK eradication MBEC values of biofilms formed by P. aeruginosa show distinct MBEC values with each device, however, both devices are equally poor in distinguishing E. coli precise BZK eradication phenotypes.
Hence, the deep well biofilm device method described herein is similarly effective for forming reproducible biofilms when compared to a standard biofilm device..

Figure 1. Schematic overview of experiment. Day 0 isolation of single colonies from cryopreserved stock; Day 1 inoculation of growth media with single colonies; Day 2 inoculation of biofilm plate; Day 3 CV staining or antimicrobial challenge; Day 4 biofilm sonication into recovery media; and Day 5 reading OD600nm of recovery plate to determine MBEC values. Some images in figure provided by Servier Medical Art (smart.servier.com). Please click here to view a larger version of this figure.

Figure 2. Example plate layouts shown for various steps of the protocol. A) The final plate setup for the initial 24 h biofilm growth using Day 1 bacterial cultures from two bacterial strains (E. coli and P. aeruginosa), each with three biological replicates. Negative control wells (grey) are used as sterility controls (no bacteria added). B) Plate setup for antimicrobial challenge of biofilms. Darkening colours indicate increasing antimicrobial concentration. Negative control are wells with no bacteria added (Grey) and positive controls are biofilms with no antimicrobial exposure (orange). Biofilm peg lids taken from panel A are transferred to this deep well plate for antimicrobial exposure. Please click here to view a larger version of this figure.
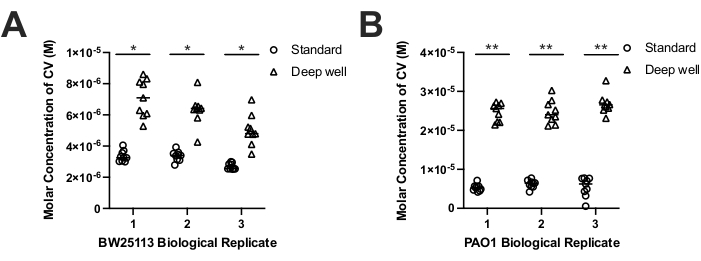
Figure 3. The molar concentration (M) of CV-stained E. coli BW25113. (A) and P. aeruginosa PAO1 (B) biofilm biomass recovered from standard (circles) and deep well (triangles) devices. CV staining was measured by reading the absorbance of CV at 550 nm (A550nm), and deep well A550nm readings were adjusted by a factor of 3.809 (800 µL/ 210 µL) to account for the volume differences between the devices. CV molar concentration (M) was determined by the Beer-Lambert law (CV Concentration =A550nm/εl); where the pathlength (l) of 210 µL in the 96 well flat bottom microtitre plate was determined to be 0.56 cm and the extinction coefficient (ε) of CV in water at A550nm of 251500 cm-1M-1 39. Results represent 9 technical replicates from three biological replicates of each strain grown as a biofilm and each biological replicate's median value is shown as a horizontal bar in each plot. Statistically significant differences in biomass between the devices was determined between biological replicate median values using a two-tailed paired t-test (E. coli p= 0.008-0.018 (*); P. aeruginosa p= 0.002-0.009 (**)) shown as bars with asterisks. Please click here to view a larger version of this figure.
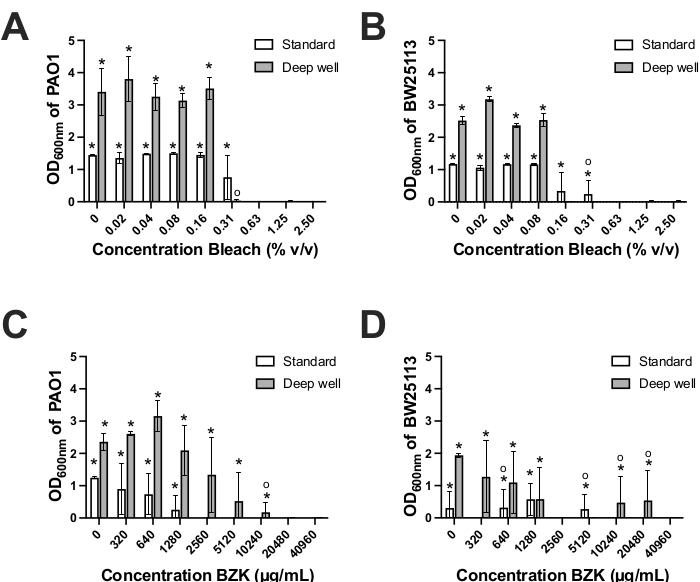
Figure 4. Determination of antimicrobial MBEC concentration. Determination of antimicrobial MBEC concentration for bleach (A,B) and BZK (C,D) for P. aeruginosa PAO1 (A, C) and E. coli BW25113 (B,D) when grown on standard (white bars) and deep well (grey bars) biofilm pegged devices. Results were determined by reading of the OD600nm of biofilm recovered after sonication into recovery media and overnight incubation. Deep well OD600nm was adjusted by a factor of 3.75 (750 µL /210 µL) to account for volume differences between the devices. Results are representative of three biological replicates and error bars show the standard deviation between replicates at each antimicrobial concentration39. Asterisks (*) above each bar plot indicate statistically significant differences in OD600nm values from blank controls with p-values < 0.05 using two-tailed Student's T-test calculations. Circle symbols (o) above each bar plot indicate measurements where only 1 or 2 replicates had statistically significant OD600nm values from blank controls. Please click here to view a larger version of this figure.
| BZK MBEC (µg/mL) | Bleach MBEC (% v/v) | |||
| Strain tested | Deep well Device | Standard Device | Deep well Device | Standard Device |
| E. coli BW25113 | 2560-40960 | 320-10240 | 0.156 | 0.625 |
| P. aeruginosa PAO1 | 20480 | 2560 | 0.313 | 0.625 |
| Deep well Device | Standard Device | p-value** | Fold change (Deep well/ Standard) | |
| Strain tested | Mean CV M*/ mm2 ± SD | Mean CV M*/ mm2 ± SD | ||
| E. coli BW25113 | 1.99 x 10-8 ± 3.25 x10-9 | 3.03 x 10-8 ± 3.31 x 10-9 | 0.0176 | -1.52 |
| P. aeruginosa PAO1 | 8.01 x 10-8 ± 9.42 x 10-9 | 5.72 x 10-8 ± 9.42 x 10-9 | 0.034 | 1.4 |
Table 1. A summary of E. coli BW25113 and P. aeruginosa PAO1 mean CV-stained biomass M/ mm2 values and biofilm eradication MBEC values for BZK and bleach on various devices.
*Mean CV M/mm2 values calculated using median biological replicate CV M values (Figure 3).
**p-values determined using Two-tailed Student's T-test.
Discussion
This study describes methods for using a larger volume 96-well high throughput static biofilm growth device involving a polypropylene deep well microplate fitted with a semi-skirted PCR-plate lid for biofilm formation (deep well biofilm device; Table of Materials). We compared biofilms generated with this device to a commercially available polystyrene standard biofilm device (Table of Materials). The deep well device method uses the same methodological steps and solutions as the standard device17 at adjusted volumes modified for the deep well devices, making this device ideal for large-scale biofilm formation and experimental analysis. The growth of E. coli BW25113 and P. aeruginosa PAO1, two Gram-negative species that are known to form biofilms, were examined for their biomass formation and disinfectant (BZK/bleach) MBEC values using on both devices. Comparison of CV-stained biomass formed on pegs from each device showed that both E. coli and P. aeruginosa formed biofilms with higher biomass on the deep well biofilm device when compared to the standard device (Figure 3A-B). Increased biofilm biomass reflected the larger surface area of deep well pegs when compared to the standard device peg surface area. When we accounted for differences in peg surface areas (mm2) with both devices, differences in biomass formation were noted, where E. coli formed significantly greater CV biomass per mm2 on polystyrene standard device pegs than with the deep well device's PCR-tube pegs (Table 1). P. aeruginosa formed greater CV-stained biomass/ peg mm2 as compared to standard devices (Table 1). These findings may highlight species-specific differences in biofilm biomass formation on the different devices.
It should be noted that bleach eradication of E. coli and P. aeruginosa biofilms on deep well devices occurred at 2-4-fold lower concentrations than standard device (Figure 4A-B, Table 1). The differences in bleach MBEC values identified from each device are likely impacted by differences in device peg shapes (deep well "pegs" are tapered tubes and standard pegs are cylindrical), plastic composition differences (polypropylene versus polystyrene), and volume differences (750 µL versus 200 µL). For example, P. aeruginosa had greater CV M biomass/ mm2 on deep well device pegs when compared to standard devices, but E. coli had less biomass on deep well pegs (Figure 3). This suggests that disinfectant concentrations required for biofilm eradication may be impacted by the biomass formed by each species as well as the available surface area. Additionally, differences in device peg shape may influence various growth conditions for particular species. In our study, P. aeruginosa may form greater biomass on deep well pegs due to their greater surface area and aeration, as this species is an obligate aerobe in contrast to E. coli, which is a facultative anaerobe. To date, we have not identified any published studies that have directly compared E. coli and P. aeruginosa biofilm formation together on both polypropylene27,28,29 and polystyrene30,31 materials. However, reports of robust E. coli and P. aeruginosa biofilm formation have been noted in independent studies examining either polypropylene or polystyrene materials. With respect to Pseudomonads, many Pseudomonas spp. can use plastics such as polypropylene as potential carbon sources32. Hence, the availability of this polypropylene deep well biofilm device is a useful advance in static biofilm studies. Polypropylene is chemically more durable than polystyrene and is a clinically relevant material, as it is frequently used in medical implants, sutures, and meshes for hernia or pelvic surgeries33,34.
Although biofilm biomass was formed by both devices, the deep well device had slightly higher variability in biomass based on the CV staining method and OD600nm biofilm eradication MBEC values for bleach and BZK. This may be explained by 3 main factors: 1) Deep well devices have greater peg surface area than standard devices that were angled as compared to standard device pegs. 2) Both species tested may have differing abilities to adhere to polypropylene and polystyrene materials of each device. 3) The volumes of growth media used in each device (750 µL deep well, 200 µL standard) and spacing between the inserted peg to the well side walls differs. These issues are not a problem if only one type of device is used for all biofilm experiments, however, if both devices are selected then the comparisons we conducted herein should be performed to identify differences36,37. Due to the differences in plastic material used in each device, the CV-stained biofilm biomass and MBEC values should not be directly compared between different devices. However, if methods and experiments are conducted on the same device (deep well or standard), the results obtained for species and antimicrobials tested are comparable.
This protocol shows that self-assembled deep well PCR-plate devices are larger volume biofilm device for measuring biofilm formation and eradication that is also cost-effective. From a cost perspective, standard biofilm devices with a 96 well pegged lids range retail at $29-36 US dollars (USD) per device (Table of Materials). Polystyrene standard biofilm devices are not autoclavable and are less tolerant to solvents/acids due to its plastic chemical properties. The self-assembled polypropylene deep well plates described herein, fitted with a separate semi-skirted 96 well PCR-plate (Table of Materials) cost a total of $14 USD per assembled device, which is half the standard biofilm device cost. It should be noted that prices may vary depending on region, distributor, and availability, and our costs after institutional discounts worked out to $9 USD/deep well device. These self-assembled deep well polypropylene PCR-plate devices have the added advantage of being autoclavable for sterilization and offer 2-4 times more biofilm biomass than standard devices.
In conclusion, this protocol and the representative findings from biofilm growth comparisons of the deep well and standard biofilm devices show that both devices are capable of cultivating bacterial biofilms, but deep well devices form 2-4 times more biofilm. The deep well biofilm device offers a viable and affordable alternative for larger volume high-throughput biofilm formation experiments such as drug susceptibility screening studies. This technique may generate biofilms useful for downstream '-omics' extractions (proteomic, metabolomic, transcriptomic) or experimental assays (enzymatic, fluorescent) that may require larger quantities of biofilm materials for analyses. The deep well biofilm device is recommended for labs that would like to study biofilms in a high-throughput 96-well assay using lower-cost, larger volume, chemically durable plastic materials that are clinically relevant.
Disclosures
The authors have no disclosures to make.
Acknowledgements
Funding for this work was provided by operating grants to DCB from the Natural Science and Engineering Research Council of Canada Discovery Grant (RGPIN- 2016-05891) and to MRM and GRG from the Government of Canadian Genomics Research and Development Initiative Grant (GRDI) program (GRDI7 2254557).
Materials
| Name | Company | Catalog Number | Comments |
| 10 mL serological pipets, individ wrap paper/plastic (200/CS) | Fisher Scientific | 13-678-11F | disposable serological pipettes for aseptic media/ culture transfer |
| 2 mL serological pipets, individ wrap paper/plastic, 500/CS | Fisher Scientific | 13-678-11C | disposable serological pipettes for aseptic media/ culture transfer |
| Axygen Aerosol Filter Tips, Sterile, 5 racks/ PK, 1000 tips/rack | Fisher Scientific | 14-222-766 | sterile pipettor tips for media aliquoting |
| Axygen deep well storage microplates, round wells, 1.1 mL cap, 5/PK, P-DW-11-C | Fisher Scientific | P-DW-11-C | microplate for 96 well deepwell device biofilm cultivation |
| Axygen Filter tips, 350 µL tips racked, 96/rack, 10 racks, low retention barrier tips | Fisher Scientific | TF-350-L-R-S | sterile pipettor tips for media aliquoting |
| Basin/reservoir natural PS 50 mL, Sterile, 5/bag, 40 bags, CS200 | Avantor/ VWR | 89094-676 | sterile basins/ reservoirs for microplate preparation |
| BD Difco Dehydrated Culture Media: Granulated Agar, 500 g | Fisher Scientific | DF0145-17-0 | materials for LB agar preparation |
| Biotek Synergy Neo2 multimode plate reader | Biotek | NEO2MB | microplate UV/Vis region plate reader |
| Branson M3800 Ultrasonic Bath, 117 V | Avantor/ VWR | CPX-952-316R | sonicating water bath |
| crystal violet (CV), ACS grade, 100 g | Fisher Scientific | C581-100 | biofilm biomass stain |
| Dimethyl sulfoxide (DMSO), 1 L, ACS grade 99.9%, poly bottle, BDH | Avantor/ VWR | CABDH1115-1LP | media components for cryopreservation |
| Easypet 3, pipet controller | Avantor/ VWR | CA11027-980 | serological pipettor for aseptic media/ culture transfer |
| Eppendorf Research Plus 8 multi-channel pipettor , 10-100 µL | Avantor/ VWR | CA89125-338 | multichannel pipettes for aseptic media/ culture transfer |
| Eppendorf Research Plus 8 multi-channel pipettor , 30-300 µL | Avantor/ VWR | CA89125-340 | multichannel pipettes for aseptic media/ culture transfer |
| Glacial acetic acid, CAS 64-19-7, 2.5L, ACS grade | Fisher Scientific | A38-212 | CV destain |
| Glass test tubes, 150 mm x 18 mm, 72/Pack, PYREX | Fisher Scientific | 14957H/ 9820-18 | materials for cell culturing |
| Glycerol, 4 L glass bottle ACS | Fisher Scientific | BP229-4 | media components for cryopreservation |
| L-Cysteine, 98%, 250 g | Avantor/ VWR | 97061-204 | universal neutralizing solution |
| L-glutathione reduced, 98%, 25 g | Fisher Scientific | AAJ6216614 | universal neutralizing solution |
| L-Hisitidine, 98%, 100 g | Avantor/ VWR | CA97062-598 | universal neutralizing solution |
| MBEC Assay Inoculator with 96 well tray, 100/CS | Innovotech | 19112 | material for 96 well MBEC device biofilm cultivation |
| McFarland Standard, 0.5 EA | Fisher Scientific | R20410 | cell culture standardization |
| McFarland Standard, 1.0 EA | Fisher Scientific | R20411 | cell culture standardization |
| NUNC 96-well microtiter plates, w/lid, 50/CS | Fisher Scientific | 167008 | microplate for 96 well MBEC device biofilm cultivation and OD measurements |
| PCR plate, semi-skirted 96 well, fast PCR, polypropylene, 25ea/PK | Sarstedt | 72.1981.202 | pegged lid for 96 well deepwell device biofilm cultivation |
| Petri dish, 100 mm x 15 mm, semi-TK CS/500 | Fisher Scientific | FB0875712 | materials for LB agar preparation |
| Potassium phosphate dibasic, ACS 500 g | Fisher Scientific | P288-500 | PBS component/ buffer |
| Sodium chloride, ACS grade, 3 kg | Fisher Scientific | S2713 | media components for LB broth and PBS |
| sodium phosphate monobasic, 1 kg | Fisher Scientific | S369-1 | PBS component/ buffer |
| Syringe filters, Sterile, PES 0.45 um, 25 mm, PK50 | Avantor/ VWR | 28145-505 | non-autoclavable solution sterilization |
| Tin foil, heavy duty, 50 feet | Grocery store | --- | materials for deepwell device sterilization |
| Tryptone (peptone from casein), 2.2 kg/EA | Fisher Scientific | B11922 | media components for LB broth |
| Tween-20, 100 mL | Fisher Scientific | BP337-100 | recovery media solution |
| Ultra-deepwell, 2.5 mL deep well plates (square well), with lid, polypropylene, 10/CS | Avantor/ VWR | 37001-520 | materials for biofilm dilution preparation |
| Yeast Extract, Fisher Bioreagents, 500 g | Fisher Scientific | BP1422-500 | media components for LB broth |
References
- Verderosa, A. D., Totsika, M., Fairfull-Smith, K. E. Bacterial Biofilm Eradication Agents: A Current Review. Frontiers in Chemistry. 7, 1-17 (2019).
- Sharma, D., Misba, L., Khan, A. U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrobial Resistance and Infection Control. 8 (1), 1-10 (2019).
- Fux, C. A., Costerton, J. W., Stewart, P. S., Stoodley, P. Survival strategies of infectious biofilms. Trends in Microbiology. 13 (1), 34-40 (2005).
- Lagacé-Wiens, P. R. S., et al. Trends in antimicrobial resistance over 10 years among key bacterial pathogens from Canadian hospitals: results of the CANWARD study 2007-16. The Journal of Antimicrobial Chemotherapy. 74, 22-31 (2019).
- Karlowsky, J. A., et al. In vitro susceptibility of urinary Escherichia coli isolates to first- and second-line empirically prescribed oral antimicrobials: CANWARD surveillance study results for Canadian outpatients, 2007-2016. International Journal of Antimicrobial Agents. 54 (1), 62-68 (2019).
- Mah, T. F. Biofilm-specific antibiotic resistance. Future Microbiology. 7 (9), 1061-1072 (2012).
- Amato, S. M., et al. The role of metabolism in bacterial persistence. Frontiers in Microbiology. 5, 1-9 (2014).
- Flemming, H. -. C., Neu, T. R., Wozniak, D. J. The EPS matrix: The "house of biofilm cells.". Journal of Bacteriology. 189 (22), 7945-7947 (2007).
- Dela Fuente-Núñez, C., Reffuveille, F., Fernández, L., Hancock, R. E. W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Current Opinion in Microbiology. 16 (5), 580-589 (2013).
- Høiby, N., et al. The clinical impact of bacterial biofilms. International Journal of Oral Science. 3 (2), 55-65 (2011).
- Merritt, J. H., Kadouri, D. E., O'Toole, G. A. Growing and analyzing static biofilms. Current Protocols in Microbiology. 01, (2005).
- Coffey, B. M., Anderson, G. G. Biofilm formation in the 96-well microtiter plate. Methods in Molecular Biology. 1149, 631-641 (2014).
- O'Toole, G. A. Microtiter dish Biofilm formation assay. Journal of Visualized Experiments. (47), e2437 (2010).
- Azeredo, J., et al. Critical review on biofilm methods. Critical Reviews in Microbiology. 43 (3), 313-351 (2017).
- Harrison, J. J., Turner, R. J., Ceri, H. High-throughput metal susceptibility testing of microbial biofilms. BMC Microbiology. 5 (53), (2005).
- Ceri, H., et al. The MBEC Assay System: Multiple Equivalent Bioffims for Antibiotic and Biocide Susceptibility Testing. Methods in Enzymology. 337, 377-385 (2001).
- Harrison, J. J., et al. Microtiter susceptibility testing of microbes growing on peg lids: a miniaturized biofilm model for high-throughput screening. Nature Protocols. 5 (7), 1236-1254 (2010).
- vanden Driessche, F., Rigole, P., Brackman, G., Coenye, T. Optimization of resazurin-based viability staining for quantification of microbial biofilms. Journal of Microbiological Methods. 98, (2014).
- Sabaeifard, P., Abdi-Ali, A., Gamazo, C., Irache, J. M., Soudi, M. R. Improved effect of amikacin-loaded poly(D,L-lactide-co-glycolide) nanoparticles against planktonic and biofilm cells of Pseudomonas aeruginosa. Journal of Medical Microbiology. 66 (2), 137-148 (2017).
- Thieme, L., et al. MBEC Versus MBIC: the Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biological Procedures Online. 21 (1), 18 (2019).
- Jennings, M. C., Ator, L. E., Paniak, T. J., Minbiole, K. P. C., Wuest, W. M. Biofilm-eradicating properties of quaternary ammonium amphiphiles: Simple mimics of antimicrobial peptides. Chembiochem. 15 (15), 2211-2215 (2014).
- Lineback, C. B., et al. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrobial Resistance & Infection Control. 7 (1), 154 (2018).
- Minbiole, K. P. C., et al. From antimicrobial activity to mechanism of resistance: The multifaceted role of simple quaternary ammonium compounds in bacterial eradication. Tetrahedron. 72 (25), 3559-3566 (2016).
- Mangalappalli-Illathu, A. K., Korber, D. R. Adaptive resistance and differential protein expression of Salmonella enterica serovar enteritidis biofilms exposed to benzalkonium chloride. Antimicrobial Agents and Chemotherapy. 50 (11), 3588-3596 (2006).
- El-Banna, T., Abd El-Aziz, A., Sonbol, F., El-Ekhnawy, E. Adaptation of Pseudomonas aeruginosa clinical isolates to benzalkonium chloride retards its growth and enhances biofilm production. Molecular Biology Reports. 46, 3437-3443 (2019).
- Ebrahimi, A., et al. Effects of benzalkonium Chloride on planktonic growth and biofilm formation by animal bacterial pathogens. Jundishapur Journal of Microbiology. 8 (2), 59764 (2015).
- Reśliński, A., et al. Evaluation of Staphylococcus aureus and Escherichia coli biofilm formation on the surface of polypropylene mesh. Medycyna doświadczalna i mikrobiologia. 63 (1), 21-27 (2011).
- Verhorstert, K. W. J., et al. In vitro bacterial adhesion and biofilm formation on fully absorbable poly-4-hydroxybutyrate and nonabsorbable polypropylene pelvic floor implants. Cite This: ACS Applied Materials & Interfaces. 12, 53646-53653 (2020).
- Arkatkar, A., Juwarkar, A. A., Bhaduri, S., Uppara, P. V., Doble, M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. International Biodeterioration & Biodegradation. 64 (6), (2010).
- Jones, J. F., et al. Oriented adhesion of Escherichia coli to polystyrene particles. Applied and Environmental Microbiology. 69 (11), 6515-6519 (2003).
- Zameer, F., et al. Evaluation of adhesive and anti-adhesive properties of Pseudomonas aeruginosa biofilms and their inhibition by herbal plants. Iranian Journal of Microbiology. 8 (2), 108-119 (2016).
- Arkatkar, A., Juwarkar, A. A., Bhaduri, S., Uppara, P. V., Doble, M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. International Biodeterioration and Biodegradation. 64 (6), 530-536 (2010).
- Dieterich, M., et al. Implant-Based Breast Reconstruction Using a Titanium-Coated Polypropylene Mesh (TiLOOP Bra). Plastic and Reconstructive Surgery. 132 (1), 8-19 (2013).
- Clavé, A., et al. Polypropylene as a reinforcement in pelvic surgery is not inert: comparative analysis of 100 explants. International Urogynecology Journal. 21 (3), 261-270 (2010).
- Zameer, F., et al. Evaluation of adhesive and anti-adhesive properties of Pseudomonas aeruginosa biofilms and their inhibition by herbal plants. Iranian Journal of Microbiology. 8 (2), 108-119 (2016).
- Chen, R., Liu, X., Han, L., Zhang, Z., Li, Y. Morphology, thermal behavior, rheological, and mechanical properties of polypropylene/polystyrene blends based on elongation flow. Polymers for Advanced Technologies. 31 (11), 2722-2732 (2020).
- Vial Loading Trays Polystyrene vs. polypropylene: Which tubes are best for your research. ChemTech International Available from: https://chemtech-us.com/polystyrene-vs-polypropylene-which-tubes-are-best-for-your-research/ (2020)
- Bock, L. J., Hind, C. K., Sutton, J. M., Wand, M. E. Growth media and assay plate material can impact on the effectiveness of cationic biocides and antibiotics against different bacterial species. Letters in Applied Microbiology. 66 (5), 368-377 (2018).
- . Crystal violet Available from: https://omic.org/spectra/PhotochemCAD/html/048.html (2017)
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved