Method Article
Screening of Coatings for an All-Solid-State Battery Using In Situ Transmission Electron Microscopy
In This Article
Summary
Utilizing the volume change of Si nanoparticles during (de)lithiation, the present protocol describes a screening method of potential coatings for all-solid-state batteries using in situ transmission electron microscopy.
Abstract
With the ever-increasing use of Li-ion batteries, especially due to their adoption in electric vehicles, their safety is in prime focus. Thus, the all-solid-state batteries (ASSBs) that use solid electrolytes instead of liquid electrolytes, which reduce the risk of flammability, have been the center stage of battery research for the last few years. However, in the ASSB, the ion transportation through the solid-solid electrolyte-electrode interface poses a challenge due to contact and chemical/electrochemical stability issues. Applying a suitable coating around the electrode and/or electrolyte particles offers a convenient solution, leading to better performance. For this, researchers are screening potential electronic/ionic conductive and nonconductive coatings to find the best coatings with suitable thickness for long-term chemical, electrochemical, and mechanical stability. Operando transmission electron microscopy (TEM) couples high spatial resolution with high temporal resolution to allow visualization of dynamic processes, and thus is an ideal tool to evaluate electrode/electrolyte coatings via studying (de)lithiation at a single particle level in real-time. However, the accumulated electron dose during a typical high-resolution in situ work may affect the electrochemical pathways, evaluation of which can be time-consuming. The current protocol presents an alternative procedure in which the potential coatings are applied on Si nanoparticles and are subjected to (de)lithiation during operando TEM experiments. The high volume changes of Si nanoparticles during (de)lithiation allow monitoring of the coating behavior at a relatively low magnification. Thus, the whole process is very electron-dose efficient and offers quick screening of potential coatings.
Introduction
Today, Li-ion batteries are all around us, from various electronic devices such as smartphones and laptops to electric vehicles, the numbers of which are steeply rising to move away from the fossil fuel-based economy1,2. With this continuously increasing, safety features of Li-ion batteries are a high priority requirement3. The liquid electrolytes that are typically used in traditional Li-ion batteries are flammable, especially at higher operating voltages and temperatures. In contrast, using nonflammable solid electrolytes in all-solid-state batteries (ASSBs) reduces the risk of flammability4. This, and potentially high energy density, has brought ASSBs to the research limelight over the last few years. However, the solid-solid electrolyte-electrode interface in ASSBs brings its own challenges that are quite different from the traditional liquid-solid electrode-electrolyte interface5. Many of the electrolytes used in ASSBs are not chemically and/or electrochemically stable against lithium and cathodes. Thus, decomposition reactions at electrode-electrolyte interfaces cause the formation of passivating layers, resulting in restricted ionic transport and an increase in internal resistance leading to capacity degradation over battery cycles6. One of the most common ways to prevent such a reaction is to apply a coating to the electrodes and/or electrolytes, which ensures there is no direct contact between the electrode-electrolyte and results in a stable interface. For this purpose, different electronic and ionic conductive coatings are currently being investigated7,8.
The main requirements for ideal coating are: it must allow ion conduction; it must not increase the battery's internal resistance; and it must be chemically and mechanically stable throughout many battery cycles. Other questions like coating thickness, single layer or multilayer, and ideal coating process are of prime interest for the commercialization of ASSBs. Thus, a screening method is needed to find out the best coatings.
A transmission electron microscope (TEM) has been used to investigate the solid-solid interface in ASSBs up to the atomic scale9,10. Furthermore, operando TEM offers the possibility to build a micro battery inside a TEM and study the battery processes during battery cycling. To track Li-ion movements in the battery, imaging at a high resolution is needed11. However, the inherent high electron beam dose of such high-resolution imaging over the entire duration of the experiment may alter the electrochemical pathways. An alternative to that is coatings that are applied on Si nanoparticles (NPs) and subjected to (de)lithiation. During operando TEM experiments, lithiation process though the coating can be monitored at low magnification, thanks to the high volume changes of Si nanoparticles during (de)lithiation12,13,14. Thus, the entire battery cycling process can be monitored at a relatively low electron dose. Further, the stress generated on the coating due to high volume changes of Si will be analogous to the stress generated on the coating over multiple cycles. Thus, long-term mechanical stability of the coatings can also be probed. This article aims to share, with examples of different thicknesses of TiO2coating, how such an operando TEM experiment can be conducted for screening the potential ASSB coatings. The protocol will explain loading the coated Si NPs on an in situ TEM holder, observing the lithiation of coated Si NPs in a TEM, and analyzing the TEM images.
Protocol
1. Preparation of TiO2 coated Si nanoparticles (TiO2@Si NPs) on half-cut TEM grids
- Prepare a half-cut TEM grid.
- Place the 3 mm TEM grids with lacey film (see Table of Materials) on a clean glass slide.
- Cut the TEM grid into half-cut grids with a razor blade.
- Drop-cast the TiO2@Si NPs on the half-cut TEM grid.
NOTE: In this study, 100 nm sized Si NPs coated with 5 nm/10 nm TiO2by atomic layer deposition15 were used. Researchers can prepare coated Si NPs in various ways.- Disperse the TiO2@Si NPs into 10 mL of acetone and dropcast onto one of the half-cut TEM grids with a pipette.
NOTE: Around 10 5 µL drops would result in sufficient TiO2@Si NPs at the edge of the half-cut TEM grid. - Check that the TiO2@Si NPs are placed at the edge via TEM.
NOTE: This is not necessary, but recommended.
- Disperse the TiO2@Si NPs into 10 mL of acetone and dropcast onto one of the half-cut TEM grids with a pipette.
- Attach tungsten (W) wire on a half-cut TEM grid.
- Cut the W wire using a nipper (see Table of Materials) into small pieces, with a length of 0.5-1 cm.
- Mix two components of conductive glue on the clean slide glass. Glue the W wire on the half-cut grid with conductive glue.
- Cure the conductive glue by drying it at room temperature in a safe place for 4 h.
NOTE: For accelerated curing, heat the specimen on a hot plate at around 100 °C for 10 min.
2. Preparing the W needle
- Cut the W wire using a nipper into small pieces, with a length of ~2 cm. Mount the W wire on the electro-polishing machine (see Table of Materials).
- Mix 50% of 1.3 mol/L NaOH and 50% of ethanol in a 10 mL beaker. Set the proper moveable range of a counter electrode to carry the electrolyte from the beaker.
NOTE: The electropolishing region can be adjusted by moving the loop up and down iteratively. The polishing region is confined to 2-4 mm by setting the range of the vertical movement of the loop. The number of vertical movements of the loop is set to five times per each trip to dip the loop in the electrolyte beaker. - Apply the voltage until the W wire is cut into two pieces-two sharp W needles.
NOTE: The polishing condition used in this study was voltage (4.0 V), and the vertical iterative movement of the loop (2-4 mm) with five iterations per electrolyte. - Load the prepared W needle on the probe head.
3. Loading the drop-casted TEM grid and W needle into the in situ TEM holder
- Insert the drop-casted half-cut TEM grid, W needle-loaded probe head, in situ TEM holder, and small glove bag (opened) into the air-free glove box (see Table of Materials).
- Scratch the Li metal with the prepared W needle (Li/LixO@W needle) probe head.
NOTE: Li is easily oxidized (Li/LixO) by a tiny amount of water. - Mount the Li/LixO@W needle probe head to the in situ TEM holder. Load the drop-casted half-cut TEM grid to the in situ TEM holder (Figure 1).
- Put the assembled in situ TEM holder into a small glove bag. Close the small glove bag and take it out of the glove box.
NOTE: Take out the assembled in situ TEM holder just before the in situ experiment so that the air contact is as low as possible.
4. Inserting the assembled in situ holder into the TEM
NOTE: The Li/LixO@W needle can be oxidized by air or water in the glove bag, so be careful.
- Seal around the empty TEM goniometer (see Table of Materials) with a large glove bag. Put the closed small glove bag containing the assembled in situ TEM holder into the large glove bag.
- Pump and purge the large glove bag with inert gas (Ar or N2) more than three times.
NOTE: The single pumping and purging process can take around a few minutes. - Open the small bag and insert the assembled in situ TEM holder. Connect the cables to the in situ TEM holder.
NOTE: One cable is for the needle movement from the control equipment, and the other is for applying the voltage or current from the power supply (see Table of Materials).
5. Performing the in situ biasing experiment in the TEM
- Align the electron beam.
NOTE: All TEM techniques and principles can be learned from reference16. - Move the Li/LixO@W needle toward the TiO2@Si NPs (Figure 2). Set the lowest magnification.
- Find the half-cut TEM grid. Locate the grid to eucentric height by the TEM goniometer. Find the Li/LixO@W needle.
- Run TEM stage wobbling. Locate the needle to eucentric height by coarse movement (inertial sliding with the repeated pulse).
NOTE: Minimization of needle movement indicates the eucentric height. - Move the needle close to the grid by coarse movement. Increase the magnification.
- Move the needle forward to the grid to make physical contact between the needle and the TiO2@Si NPs by fine movement (piezoelectric tube).
NOTE: Contrast change of the TiO2@Si NPs indicates physical contact.
- Set proper magnification and beam intensity.
NOTE: The electron dose rate used in this study was 10 e-/Å2/s, a comparable condition for a biological sample. - Apply voltage and capture the image or video.
NOTE: The voltage used in this study was 2 V.
6. Analyzing the TEM images
- Load the TEM image. Draw a polygon to target particle.
- Measure the area of the drawn polygon. Compare the measured area among various TEM images.
NOTE: For the quantification purpose, setting the scale (unit: pixel per length) is required before the measurement. ImageJ (see Table of Materials) was used for processing the images in the present study.
Results
A series of TEM images of lithiation on 5 nm and 10 nm TiO2coated Si/SiO2particles are shown in Figure 3. In the case of 5 nm coating, significant expansion occurred in the whole area, and the coating was not broken during huge expansion. In the case of 10 nm coating, relatively small expansion occurred even for a longer lithiation time, and the coating was broken after 2 min. From the amount of expansion and coating breakage, 5 nm coating is promised to show better capacity and durability than 10 nm coating.
The amount of particle expansion can be obtained by image processing as shown in Figure 4. The 5 nm coating case showed around 2x areal expansion, while the 10 nm coating case showed only 1.2x areal expansion. The expansion rate of the 5 nm coating case is six times faster than that of the 10 nm coating case.
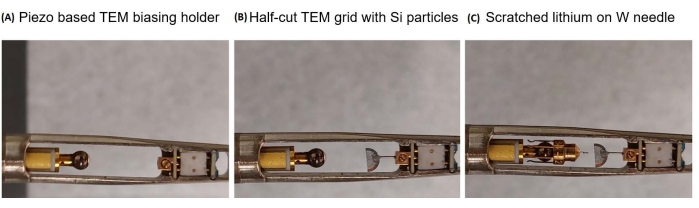
Figure 1: In situ TEM holder assembly. (A) An empty in situ TEM biasing holder. (B) Assembling the drop-casted half-cut TEM grid with a tungsten rod to the right side of the holder. (C) Assembling the probe head with a tungsten needle to the left side of the holder. Please click here to view a larger version of this figure.

Figure 2: Moving the tungsten needle toward the TiO2 coated Si nanoparticles in TEM. (A) Locating the tungsten needle to the eucentric height and moving the needle close to the TEM grid. (B) Physical contact between the needle and nanoparticles is indicated by contrast change. Please click here to view a larger version of this figure.
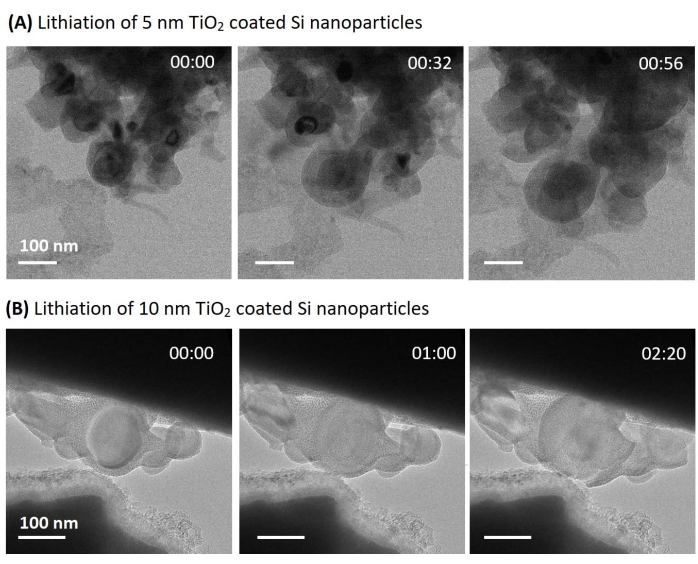
Figure 3: TEM image series about lithiation. (A) 5 nm TiO2 coated Si nanoparticles. (B) 10 nm TiO2 coated Si nanoparticles. The figure is adapted from Basak et al.15. Please click here to view a larger version of this figure.
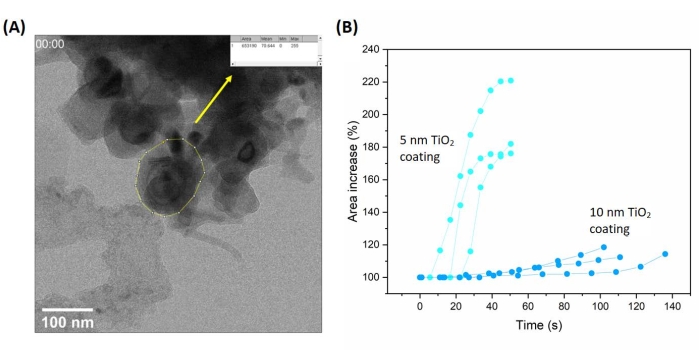
Figure 4: Tracking the expansion of nanoparticles during lithiation. (A) Measuring the area of the nanoparticles (by a drawn polygon) from the TEM image. (B) The graph of area increase vs. time. The figure is adapted from Basak et al.15. Please click here to view a larger version of this figure.
Discussion
The lithiation of coated Si NPs via in situ TEM enables simple examination of the potential coatings for ASSBs. One of the important steps in determining the success of these experiments is the appropriate thickness of LiOx, which acts as a solid electrolyte in these experiments. As the ionic conductivity of LiOx is significantly lower than that of the typical solid electrolyte used in ASSBs, a thicker LiOx layer would increase internal resistance and hamper ion conduction. On the other hand, any non-oxidized area of lithium may act as an optional means of battery short circuit. The appropriate thickness of LiOx can be ensured by carefully transporting the assembled holder from the glovebox to the TEM using the so-called glove bag (described in steps 3 and 4).
The coating behavior during the lithiation can be investigated in a more in depth manner, even at this low magnification if the coating data (signal) is extracted separately from TEM images without the data of Si-core (noise). Before the lithiation, coating and Si NPs are easily distinguished by the contrast. However, during the lithiation, the contrast difference was decreased, so it was hard to investigate the phenomena of coating independently. STEM imaging can enhance the contrast, and the intensity of STEM images can be used for volume measurement. Furthermore, machine learning or deep learning technology can enhance feature recognition and extract more information to understand the mechanisms during the in situ experiments17.
The current procedure of (de)lithiation of coated Si NPs via in situ TEM is limited to quick screening to find the potential coating materials. The shortlisted coating candidates must be tested in the actual ASSBs. In situ biasing studies of the micro batteries, prepared by focused-ion-beam on a microelectromechanical system (MEMS), can provide further information on the interfacial ionic transport mechanism6,11.
This coating screening technique can be adapted to Na-ion based ASSBs by replacing the lithium with sodium.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is conducted in the framework of "Electroscopy" (grant no. 892916) from the Marie Sklodowska-Curie action. J.P., O.C., H.T., and H.K., acknowledge the project iNEW FKZ 03F0589A from BMBF. CG acknowledges funding from the Royal Society, London for a URF (Grant no. UF160573).
Materials
| Name | Company | Catalog Number | Comments |
| 3 mm TEM grids with lacey film | Ted Pella | ||
| Acetone | Sigma Aldrich | ||
| Ar gas | Linde | ||
| Conductive glue | Chemtronics | CW2400 | |
| Electro-polishing machine | Simplex Scientific LLC | ElectroPointer | Including counter electrode (a small loop made by Platinum) |
| Ethanol | Sigma Aldrich | ||
| Glove bag | |||
| Glove box | |||
| Image Processing program | ImageJ | ||
| In-situ biasing TEM holder | Nanofactory | Nanofactory STM-TEM holder | Including piezo control equipment |
| NaOH | Sigma Aldrich | ||
| Nipper | |||
| Power supply | Keithley | ||
| TiO2 coated Si/SiO2 particles | In house made, TiO2 coated on commerical Si nanoparticles by atomic layer deposition method | ||
| Transmission electron microscope (TEM) | ThermoFisher Scientific | Titan G2 | |
| Tungsten (W) wire (diameter: 0.25 mm) | any available brand |
References
- Armand, M., Tarascon, J. -M. Building better batteries. Nature. 451 (7179), 652-657 (2008).
- Goodenough, J. B., Park, K. -S. The Li-ion rechargeable battery: a perspective. Journal of the American Chemical Society. 135 (4), 1167-1176 (2013).
- Liu, K., Liu, Y., Lin, D., Pei, A., Cui, Y. Materials for lithium-ion battery safety. Science Advances. 4 (6), (2018).
- Grey, C. P., Hall, D. S. Prospects for lithium-ion batteries and beyond-a 2030 vision. Nature Communications. 11 (1), 6279(2020).
- Basak, S., et al. Operando transmission electron microscopy study of all-solid-state battery interface: redistribution of lithium among interconnected particles. ACS Applied Energy Materials. 3 (6), 5101-5106 (2020).
- Wang, L., et al. In-situ visualization of the space-charge-layer effect on interfacial lithium-ion transport in all-solid-state batteries. Nature Communications. 11 (1), 5889(2020).
- Lee, D. J., et al. Nitrogen-doped carbon coating for a high-performance SiO anode in lithium-ion batteries. Electrochemistry Communications. 34, 98-101 (2013).
- Wu, E. A., et al. A facile, dry-processed lithium borate-based cathode coating for improved all-solid-state battery performance. Journal of The Electrochemical Society. 167 (13), 130516(2020).
- Liu, Y., et al. Visualizing the sensitive lithium with atomic precision: cryogenic electron microscopy for batteries. Accounts of Chemical Research. 54 (9), 2088-2099 (2021).
- Sheng, O., et al. Interfacial and ionic modulation of poly (ethylene oxide) electrolyte via localized iodization to enable dendrite-free lithium metal batteries. Advanced Functional Materials. 32 (14), 2111026(2022).
- Gong, Y., et al. In situ atomic-scale observation of electrochemical delithiation induced structure evolution of LiCoO2cathode in a working all-solid-state battery. Journal of the American Chemical Society. 139 (12), 4274-4277 (2017).
- Huang, J. Y., et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science. 330 (6010), 1515-1520 (2010).
- Liu, X. H., et al. Anisotropic swelling and fracture of silicon nanowires during lithiation. Nano Letters. 11 (8), 3312-3318 (2011).
- Liu, X. H., et al. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano. 6 (2), 1522-1531 (2012).
- Basak, S., et al. Operando transmission electron microscopy of battery cycling: thickness dependent breaking of TiO 2 coating on Si/SiO 2 nanoparticles. Chemical Communications. 58 (19), 3130-3133 (2022).
- Williams, D. B., Carter, C. B. Transmission Electron Microscopy. , Springer. US. Boston, MA. (2009).
- Horwath, J. P., Zakharov, D. N., Mégret, R., Stach, E. A. Understanding important features of deep learning models for segmentation of high-resolution transmission electron microscopy images. npj Computational Materials. 6 (1), 108(2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved