Method Article
Continuous Venous-Arterial Doppler Ultrasound During a Preload Challenge
In This Article
Summary
The Frank-Starling-Sarnoff curve is clinically important and describes the relationship between cardiac preload and output. This report illustrates a novel method of simultaneous jugular venous and carotid arterial Doppler velocimetry as transient surrogates of cardiac preload and output, respectively; this approach is enabled by wireless, wearable Doppler ultrasound.
Abstract
A preload challenge (PC) is a clinical maneuver that, first, increases the cardiac filling (i.e., preload) and, second, calculates the change in cardiac output. Fundamentally, a PC is a bedside approach for testing the Frank-Starling-Sarnoff (i.e., "cardiac function") curve. Normally, this curve has a steep slope such that a small change in the cardiac preload generates a large change in the stroke volume (SV) or cardiac output. However, in various disease states, the slope of this relationship flattens such that increasing the volume into the heart leads to little rise in the SV. In this pathological scenario, additional cardiac preload (e.g., intravenous fluid) is unlikely to be physiologically effective and could lead to harm if organ congestion evolves. Therefore, inferring both the cardiac preload and output is clinically useful as it may guide intravenous (IV) fluid resuscitation. Accordingly, the goal of this protocol is to describe a method for contemporaneously tracking the surrogates of cardiac preload and output using a novel, wireless, wearable ultrasound during a well-validated preload challenge.
Introduction
At its foundation, the Frank-Starling-Sarnoff curve describes the relationship between cardiac preload and output1,2,3,4. Historically, this curve is depicted by plotting the right atrial pressure on the abscissa and the cardiac output or stroke volume (SV)5 on the ordinate. Assessing the slope of this curve is clinically important because the relationship between cardiac filling and output is dynamic; thus, the slope of the curve informs the resuscitation strategy1,4. Specifically, if the slope of the Frank-Starling-Sarnoff (i.e., "cardiac function") curve is steep, then increasing the preload (e.g., administering intravenous fluid) augments the output. By contrast, if the slope of the cardiac function curve is shallow, then providing intravenous (IV) fluid does not increase the SV2.
Knowing when IV fluid does or does not increase the SV is important so that the treating clinician can avoid physiologically ineffective fluid4,6, in other words, the scenario in which giving IV fluid to a patient does not increase the SV7,8. Identifying this relatively common clinical state is achieved via a preload challenge (PC), which is a clinical maneuver that "tests" the slope of the cardiac function curve3. A PC is achieved by rapidly increasing the cardiac filling and measuring the change in SV9. As above, IV fluid can act as a PC, as can gravitational maneuvers such as moving the head below the level of the heart (i.e., Trendelenburg positioning)10 or moving from a semi-recumbent position to supine with the legs elevated (i.e., a passive leg raise)11. In fact, the passive leg raise (PLR) is a well-accepted and well-validated PC that is employed in modern intensive care units and recommended by experts prior to IV fluid administration during sepsis resuscitation4,12. Importantly, it is suggested that during the PLR, the clinician should measure both the cardiac preload (e.g., the change in right atrial pressure) and the output (e.g., the change in SV) to adequately test the cardiac function curve13. However, the former is rarely performed as simultaneous measures are cumbersome and an invasive catheter placed into the right atrium is often required.
Ultrasonographic surrogates of cardiac filling and output have grown in popularity over the last few decades, especially in emergency departments and intensive care units2,14. Specifically, the simultaneous assessment of both a great vein and large artery acts as a surrogate for cardiac preload and output, respectively2,15. For example, morphological changes in great vein Doppler have been found to track right atrial pressure-this is true for the internal jugular16,17,18, hepatic, and portal veins19, superior vena cava20, inferior vena cava21, femoral veins22, and even intrarenal veins23. Thus, great vein Doppler velocimetry operates as a surrogate for cardiac filling2. However, the Doppler of a large artery can transiently track changes in cardiac output. For instance, measures of common carotid artery systolic time24,25, velocity26,27,28, and flow 29,30 have shown promise for detecting SV changes.
A novel, wireless, wearable, continuous wave Doppler ultrasound that simultaneously insonates both the internal jugular vein and common carotid artery has previously been described14,15,27,28,31,32,33,34,35,36. Herein, a method using this device during a commonly employed, clinical PC-the passive leg raise-is illustrated. Further, the internal jugular and common carotid arterial Doppler morphologies during the PC are described as possible surrogates of cardiac preload and output, respectively. This protocol is clinically important because it provides both a practical and physiological foundation for future patient study. For example, inpatients (e.g., perioperative setting, sepsis, critically ill) and outpatients (e.g., congestive heart failure, dialysis) could be monitored by the method, or modifications thereof, described below.
Protocol
When performing a preload challenge using the wireless, wearable Doppler ultrasound system, there are a number of critical steps that the user should consider. Written and informed consent was obtained for this protocol; the study was reviewed and approved by the Research Ethics Board of Health Sciences North. The procedures followed were in accordance with the local ethical standards of the committee on human experimentation and with the Helsinki Declaration of 1975.
1. Identifying an appropriate patient
- Identify a patient on whom the wearable Doppler ultrasound device will be placed. Ensure that the patient is calm and relatively motionless to minimize phonation and deglutition for the duration of the assessment (1-5 min).
- Position the patient in the semi-recumbent or semi-Fowler position in the hospital bed or gurney. Specifically, adjust the bed such that the torso is at an angle of 30-45° above horizontal.
2. Obtaining the carotid artery and internal jugular Doppler signals
- Turn on the wearable Doppler ultrasound by pressing the round button in the center of the ultrasound device. Blue lights around the periphery of the button will flash, signaling that the device is on and ready to pair with a smart device.
- Turn on the dedicated application on the smart device. Press the start button on the smart device application. Observe the list displayed on the application showing the discoverable, wearable, ultrasound devices within physical proximity of the smart device. Match the number affixed to the face of the desired ultrasound device to the indicated device on the application list. Press connect to pair the desired ultrasound device to the application.
- Confirm the desired ultrasound device is paired by observing white flashing lights around the button in the center of the device. Press correct on the smart device application to complete the pairing.
- Apply a small amount of ultrasound gel to the large face of the transducer wedge on the back of the ultrasound device.
NOTE: The gel application produces a characteristic Doppler signal artifact, which can be seen on the smart device application. - Tap the large face of the transducer wedge to ensure the device is live and paired to the smart device application. Ensure that the volume on the smart device application is turned on by pressing the volume icon button in the top-right corner of the application display.
- With the patient's neck slightly extended, note the laryngeal prominence, and hold the ultrasound device so that the large face of the transducer wedge faces downward toward the patient's heart. Place the wedge of the device on the lateral aspect of the patient's laryngeal prominence. Look for an audio and visual response on the smart device application: the top portion of the application will display a waveform spectrum for the carotid artery and jugular vein. The bottom portion of the application quantifies the corrected flow time (ccFT) for each cardiac cycle, displayed as green bars.
- Slide the transducer face on the patient's neck laterally from a perpendicular plane defined by the trachea until the carotid Doppler spectrum is detected both visually and audibly on the smart device application.
NOTE: In most patients, the audio and visual Doppler spectra of the carotid artery and jugular vein are detected within a few centimeters of the lateral laryngeal border.
3. Optimizing the carotid artery and internal jugular Doppler signals
- While holding the device in place, observe the carotid Doppler spectrum and its features on the top of the application display. A good carotid artery Doppler signal is identified by its characteristic sharp velocity upstroke with a good signal-to-noise ratio and a clear dicrotic notch, which demarcates the end of mechanical systole. The application will automatically begin tracing the Doppler spectrum once a strong enough signal is obtained, indicated by a white line around the maximum of the waveform.
- While holding the device in place, observe the velocity measurements using the scale on the top left-hand side of the smart device display. Using the auto-trace over the carotid artery maximum, ensure that the trace is in a typical range. The peak systolic velocity of the carotid artery is typically between 50 cm/s and 120 cm/s, and the end diastolic velocity is typically less than 20 cm/s.
- Slowly slide the ultrasound device laterally slightly by a few millimeters while looking at the dicrotic notch on the artery spectrum to ensure that a clear velocity nadir is observed reliably. If the dicrotic notch velocity becomes difficult to see, repeat this step, but slide the ultrasound device medially.
- Repeat steps 3.1-3.3 over the contralateral carotid artery to assess for the presence of a clearer dicrotic notch velocity.
- After observing for the presence of a clear dicrotic notch velocity on both carotid arteries, select the side of the neck to which the device will be adhered. Choose the side with the most obvious dicrotic notch velocity. If both sides of the neck have equally acceptable dicrotic notch velocities, choose the side of the neck with the most robust internal jugular Doppler spectrum.
4. Adhering the ultrasound device to the neck
- Prepare to adhere the device to the chosen carotid artery by visually noting where on the neck the best signal was obtained. If needed, use a skin-marking pen to identify the optimal placement position. Lift the device from the neck, and remove the protective backing from the adhesive attached to the ultrasound device.
- Observe the transducer face on the ultrasound device, and determine if there is a sufficient amount of ultrasound gel remaining. If needed, reapply a small amount of ultrasound gel to the transducer face. Remove excess ultrasound gel from the neck that may have remained during signal discovery as this may interfere with the adhesion of the device.
- Return the device to the neck to the location identified in step 4.1, with the large face of the transducer wedge pointing downward toward the heart. Smooth the wings of the adhesive across the neck. Remove the protective backing from the tips of the adhesive after pulling tight; place the filming against the skin to fully secure the device to the neck. Monitor the carotid and jugular spectra throughout adhesion to ensure that the signal is not lost.
5. Performing a preload challenge via a passive leg raise (PLR)
- Ensure that the patient is in the semi-recumbent position on the hospital bed or gurney, as identified in step 1.2.
- Clear the smart device application data by pressing restart on the smart device application. Press begin assessment on the smart device application to obtain the baseline measures for the passive leg raise (PLR). Begin with 30-60 s of resting baseline with the patient in the semi-recumbent position on the hospital bed or gurney. Look for a marker displayed on the bottom portion of the application display to signify the beginning of the assessment.
- Prepare the necessary measures to perform a PLR (e.g., obtain extra nursing help as needed).
- Once ready to perform a PLR, press mark intervention on the smart device application to signify the beginning of the preload challenge (in this case, a PLR). Look for a marker displayed on the bottom portion of the application display to signify the beginning of the intervention. Perform a PLR; without touching the patient, reposition the hospital bed or gurney so that the torso is moved downward to the horizontal and the legs are lifted to 30-45° above the horizontal.
NOTE: The user must take great care to keep the patient fully passive during this maneuver. - Keep the patient in the PLR position for 90-120 s.
NOTE: Throughout the maneuver, it is imperative that the patient keep their neck completely still so as not to change the insonation angle between the transducer face and the vessels in the neck. If needed, manually stabilize the patient's neck. - Observe the jugular Doppler spectrum on the smart device application during the intervention; assess for changes in the absolute jugular venous velocity and its pattern as a surrogate for the jugular venous pressure.
- Observe the evolution of the green bars on the smart device application during the intervention; assess for changes in the ccFT before and after the start of the preload challenge. The smart device application automatically quantifies the ccFT for each cardiac cycle and represents this as a green bar.
- Once the intervention is complete, press end assessment on the smart device application. Look for a marker that will be displayed on the bottom portion of the application display to signify the end of the assessment.
- Return the patient back to the baseline, semi-recumbent position.
- If desired, press save on the smart device application to save the assessment and export the data files (see additional data notes for more details).
6. Observing the changes in the carotid corrected flow time (ccFT) on the smart device application following the completed assessment
- Observe the assessed changes in the ccFT displayed in a yellow box on the lower-right side of the application.
NOTE: The smart device application automatically quantifies the changes in ccFT between the recorded baseline measurements and the preload challenge/intervention measurements. - Press save on the application, and wait for the data to be split into the following files: two .txt format files containing IQ and Tick data from the Doppler device hardware; one PKL format file containing the spectrogram information (use this to visualize the real-time collected data online); and two .json format files containing the session information (such as the date and time, smart device hardware settings, user settings, and more) and real-time calculations per cardiac cycle.
Results
With respect to interpreting the continuous venous-arterial Doppler ultrasound during a preload challenge, general physiological responses are illustrated in Figure 1, Figure 2, Figure 3, and Figure 4.
First, in a patient with a normal, upright cardiac function curve, a small increase in the cardiac preload (e.g., as inferred by jugular venous Doppler) is accompanied by a relatively large rise in the stroke volume (e.g., as indicated by ccFT augmentation)2,14,36; this is exemplified by Figure 1. Inferring changes in the jugular venous pressure (JVP) from the jugular Doppler spectrum during the preload challenge deserves some elaboration. Again, this physiological variable is a surrogate for cardiac preload or filling. Normally, the jugular vein is collapsed in the upright position when the jugular venous pressure is less than the atmospheric pressure. In the Doppler spectrum, this translates to a relatively high velocity (i.e., usually more than 50 cm/s) with minimal pulsations and low amplitude (i.e., the intensity or "brightness" of the jugular signal). Then, if the jugular venous pressure rises during the maneuver, the vein rounds out in diameter, its velocity falls (i.e., usually to less than 50 cm/s), the intensity (i.e., "brightness") increases, and the waveform becomes more pulsatile2,14,36. As shown in Figure 1, the change in the venous Doppler morphology indicates that the jugular vein has increased in diameter (i.e., falling velocity, rising amplitude) and is beginning to follow the right atrial pressure deflections. Though not pictured, with increased right atrial pressure, the "v" wave during late systole can cleave the monophasic wave seen in Figure 1 into a systolic "s" velocity wave and a diastolic "d" velocity wave2,14,36. In as-of-yet unpublished data in healthy volunteers, we observed that jugular venous Doppler morphology was the most accurate venous ultrasonographic measure for distinguishing low from high preload states.
In contrast, an abnormal response is depicted in Figure 2. A clinical example of this pathophysiology is a hypovolemic, veno-dilated, septic patient with evolving septic cardiac dysfunction2,15,36. Such a patient has diminished venous return (which reduces the cardiac preload, i.e., the right atrial or jugular venous pressure) and simultaneously depressed cardiac function2,15,35,36. Therefore, at baseline, this patient demonstrates a continuous, low-JVP venous Doppler morphology that increases (i.e., becomes more pulsatile) during the preload challenge without a significant rise in the ccFT. This effectively describes a flattened slope of the cardiac function curve.
The results from continuous venous-arterial Doppler could also alert the treating clinician to problems with the PLR itself. For example, in some situations, the PLR may not recruit enough venous blood from the lower extremities and splanchnic circulation to generate a physiologically effective preload challenge4. Without assessing the cardiac filling, this could result in a "false negative" PLR. However, if the clinician sees little ccFT response (i.e., as a stroke volume surrogate) coupled with no change in the venous Doppler (i.e., as a surrogate for preload), this could herald an ineffective PLR, as seen in Figure 3.
Lastly, it is critical that the PLR maneuver is true to its namesake, meaning that there is no exertion by the patient when the torso falls and the legs elevate13. This avoids adrenergic discharge, which may increase the cardiac function independently of the venous return; however, as described in Figure 4, this undesired scenario may be indicated by the parameters of a rising stroke volume in the arterial signal coupled with a venous Doppler morphology, suggesting diminished venous pressure.
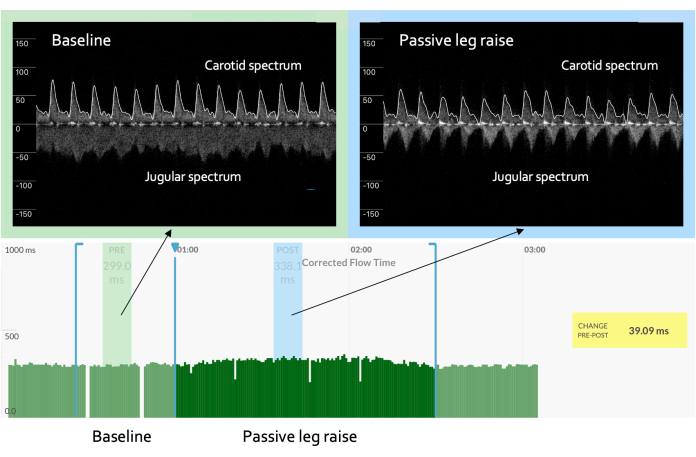
Figure 1: Increased slope of the cardiac function curve. In an example of a "normal" or "expected" result, the venous waveform progresses from being high velocity, low amplitude, and non-pulsatile to being lower velocity, higher amplitude, and pulsatile in character. The pulsatile venous waveform can be marked by a monophasic signal, as seen here. Concomitantly, the arterial Doppler waveform shows an increase in the ccFT from baseline, suggesting that the increase in the cardiac preload is met by a rising cardiac output. These responses, taken together, indicate a "cardiac function" curve with a steep slope. The y-axis on the spectrum represents the velocity in centimeters per second. The positive velocity is toward the brain (e.g., the carotid artery), while the negative velocity is toward the heart (e.g., the jugular velocity). The x-axis on the spectrum is time. Please click here to view a larger version of this figure.
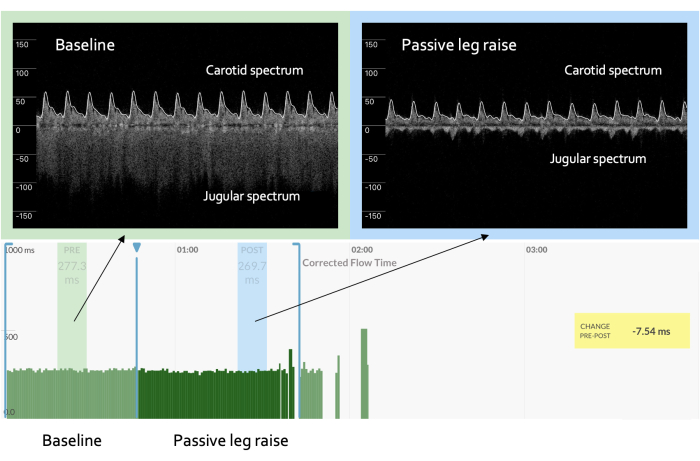
Figure 2: Flattened slope of the cardiac function curve. An "abnormal" response during a preload challenge is marked by a venous Doppler waveform that evolves as above but with an arterial response that reveals no significant change or even a decrease in the ccFT as compared to baseline, as seen here. This constellation of venous and arterial findings implies a flat or, potentially, impaired cardiac function curve with increased preload. Please click here to view a larger version of this figure.
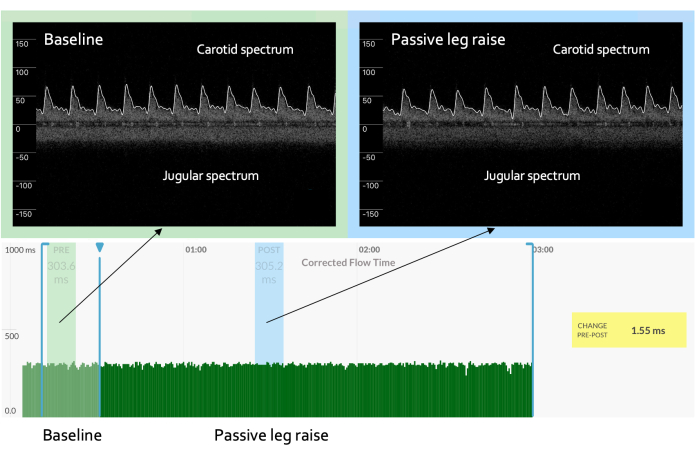
Figure 3: No change in the venous Doppler. A preload challenge that shows no significant change in the venous Doppler waveform could represent an inadequate change in cardiac filling, meaning no change in the arterial spectrum is expected. Please click here to view a larger version of this figure.
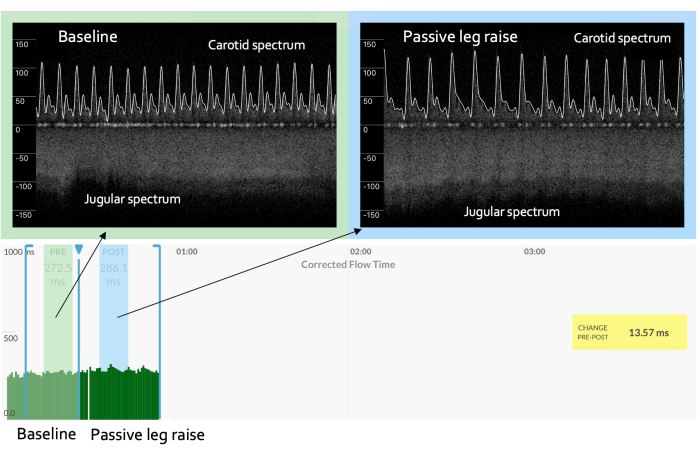
Figure 4: Falling preload during a preload challenge. A preload challenge that shows rising venous velocity and a significant increase in arterial Doppler measures may mean augmented adrenergic tone (i.e., sympathetic stimulation) such that the cardiac function increases independently of the venous return. This circumstance could be the result of a "non-passive" leg raise, for example, if the patient strains to change their body position. Please click here to view a larger version of this figure.
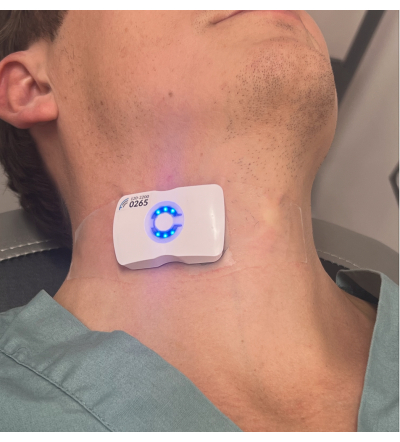
Figure 5: The device on a volunteer. Please click here to view a larger version of this figure.
Discussion
The main purpose of this visual experiment is to describe a protocol for contemporaneously tracking the surrogates of cardiac preload and output during a well-validated PC using a wireless, wearable ultrasound. The goal is not to describe a specific study protocol in patients, per se. However, the description of continuous venous and arterial Doppler serves as a practical and physiological foundation for designing studies in patients both in need of resuscitation (e.g., perioperative period, sepsis) or de-resuscitation (e.g., congestive heart failure, dialysis, failure to liberate from mechanical ventilation)15,36.
The method described employs a wearable, continuous wave Doppler ultrasound that simultaneously insonates a major vein and artery to infer the cardiac function during a PC15. Critical to this method is the selection of an appropriate, cooperative patient and ensuring a minimal angle change between the vessels and the transducer throughout the assessment. Furthermore, assuring a clear and consistent dicrotic notch velocity is paramount to allow for the consistent measurement of the systolic time. Finally, the user must appreciate the venous Doppler morphology and its variation across a spectrum of jugular venous pressure (JVP), as discussed above in the representative results.
As a modification to the method described, instead of a PLR, the PC might consist of a rapid infusion of intravenous fluid9, moving a completely supine patient from horizontal to head down by 15-30° (i.e., Trendelenburg positioning)10, or respiratory maneuvers such as end-expiratory occlusion34. These approaches are beneficial in that there is less patient movement and, ostensibly, a reduced risk of angle change during the assessment. In general, troubleshooting all PCs with the wearable ultrasound requires stable neck positioning, extra adhesive to secure the insonation angle, the prolongation of the assessment when phonation or deglutition artifacts occur, the repositioning of the device, or the addition of ultrasound gel to optimize the acoustic coupling to the patient31.
There are limitations to the method of cardiovascular inference described within this manuscript. With regard to the jugular venous signal, the Doppler morphology is a surrogate of the jugular venous pressure, which itself is a surrogate of the right atrial pressure37,38,39,40. Therefore, there is no certainty that the cardiac preload is increased based on the venous Doppler changes alone. Nevertheless, the venous Doppler waveform varies its morphology based upon the pressure deflections of the right atrium17,18,41; this has been observed in multiple great veins in addition to the jugular. For example, evaluations of the superior and inferior vena cava and the hepatic, portal, intrarenal, and femoral veins all qualitatively estimate the venous pressure42. More specifically, the prominent venous velocity wave during systole is formed by the x-descent of the right atrial pressure and the diastolic velocity wave by the y-descent of the right atrial pressure. The velocity nadir between systole and diastole is due to the right atrial pressure "v wave"16,17,18,42.
Additionally, while the duration of mechanical systole is directly proportional to the stroke volume, the systolic time, similar to SV, is mediated by the heart rate, preload, afterload, and contractility43. While the ccFT equation corrects for heart rate, a limitation of the ccFT as a surrogate for the stroke volume is that it is determined by other hemodynamic inputs. Nevertheless, increases in the ccFT by at least 7 ms24 or by +2%-4% have been shown to accurately detect a 10% rise in the SV in critically ill patients24, healthy volunteers performing a preload modifying maneuver44,45, and healthy volunteers undergoing simulated moderate-to-severe hemorrhage resuscitation27. Furthermore, ccFT has been used to accurately track changing SVs in the elective surgical population during respiratory maneuvers46. Thus, assuming that afterload and contractility are relatively constant during a focused PC, the ccFT varies primarily due to changes in the SV.
Furthermore, the absolute and relative contraindications for this approach have yet to be elaborated, especially in patients. As noted above, the most common contraindication is likely an inability to cooperate (e.g., delirious, speaking, movement, rigors). This is true for many modern vital sign monitors, though the wearable ultrasound is particularly sensitive to phonation and neck movement. Accordingly, the device works very well in intubated and paralyzed patients in the operating room; a study using the device on patients receiving elective coronary artery bypass grafting is currently enrolling. Physiological variation between the opposing carotid arteries in a particular patient is possible; however, this concern is mitigated because, in the PC paradigm, the patient acts as their own control (i.e., a pre-post intervention). Accordingly, we anticipate that while the different sides of the neck (Figure 5) may produce slightly different venous and arterial Doppler signals, the change should be consistent barring any significant unilateral abnormalities (e.g., stenosis). Physical limitations may also pose problems (e.g., central lines, cervical-spine collars, tracheotomy straps, trauma, short necks, or severe cervical kyphosis). Physiological contraindications such as moderate-to-severe carotid stenosis, aortic stenosis, arrhythmia, and abnormal respiratory patterns are also of potential concern. Generally, however, a PLR with real-time measures of cardiac output is resistant to many of these issues, including arrhythmia4,11. The device is currently being studied in both spontaneously breathing emergency department patients and in the operating room; the proportion with unusable signals will be gleaned from this data.
The significance of the method described above is that the adhered ultrasound can sample minutes of continuous data, while hand-held approaches are typically limited to a few cardiac cycles48,49. Additionally, the software for the wearable ultrasound measures the arterial Doppler coefficient of variation. From this, a "smart window" is implemented to sample a sufficient number of cardiac cycles at baseline and during the intervention; this statistical instrument tailors the measurement precision for each preload challenge47. Moreover, given that the wearable ultrasound remains affixed to the patient, the risk of human factors50,51 that increase the measurement variability is diminished; this holds for both arterial and venous insonation. Another significant aspect of this method is that contemporaneous venous and arterial Doppler assessment allows the clinician to indirectly assess the cardiac preload during a dynamic maneuver; this is recommended by experts in the field13 but rarely performed because measuring the right atrial pressure is cumbersome. Accordingly, continuous venous-arterial Doppler during a PC gives a deeper picture of the cardiac function at the bedside. While this method described above may be used to judge intravenous fluid resuscitation, it also holds promise for gauging "de-resuscitation"15,52 or predicting weaning from mechanical ventilation53 and should be explored in future clinical research. For example, the diuresis of patients with volume overload may be revealed by signs of falling right atrial pressure within the venous Doppler signal as the volume removal progresses. Further, should the patient receive a PLR before and after dialysis, the change in arterial Doppler measures should indicate increased cardiac function, as previously reported52.
A method of continuous venous-arterial Doppler during a PC is best accomplished by following the six general steps outlined above in the protocol section. A novel, wireless, wearable Doppler ultrasound system assists this paradigm by adhering to a patient and enabling a relatively fixed insonation angle during the preload change. Fundamentally, simultaneous, instantaneous, venous-arterial Doppler may elaborate the two axes of the Frank-Starling-Sarnoff relationship and, therefore, give new insights into cardiac function. This is especially important when managing acutely ill patients; both volume administration and removal could be refined by this new approach. While the above discussion is largely limited to inpatient applications, additional outpatient uses within the spheres of congestive heart failure, chronic renal failure, and pulmonary hypertension are also possibilities. Accordingly, continuous venous-arterial Doppler may unlock unforeseen channels of exploration within hemodynamics and related medical disciplines.
Disclosures
J.E.S.K., S.O.G., D.J., L.M.H., E.R., G.C., J.K.E. work for Flosonics Medical, the start-up that builds the wearable Doppler ultrasound; R.A. and B.N. declare no competing interests.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| FloPatch | Flosonics | ||
| iPad | Apple | ||
| ultrasound gel |
References
- Berlin, D. A., Bakker, J. Starling curves and central venous pressure. Critical Care. 19 (1), 55 (2015).
- Kenny, J. -. E. S. Assessing fluid intolerance with Doppler ultrasonography: A physiological framework. Medical Sciences. 10 (1), 12 (2022).
- Monnet, X., Marik, P. E., Teboul, J. -. L. Prediction of fluid responsiveness: An update. Annals of Intensive Care. 6 (1), 111 (2016).
- Monnet, X., Shi, R., Teboul, J. -. L. Prediction of fluid responsiveness. What's new. Annals of Intensive Care. 12 (1), 46 (2022).
- Kenny, J. -. E. S., Barjaktarevic, I. Letter to the editor: Stroke volume is the key measure of fluid responsiveness. Critical Care. 25 (1), 104 (2021).
- Malbrain, M. L., et al. Principles of fluid management and stewardship in septic shock: It is time to consider the four D's and the four phases of fluid therapy. Annals of Intensive Care. 8 (1), 66 (2018).
- Douglas, I. S., et al. Fluid response evaluation in sepsis hypotension and shock: A randomized clinical trial. Chest. 158 (4), 1431-1445 (2020).
- Latham, H. E., et al. Stroke volume guided resuscitation in severe sepsis and septic shock improves outcomes. Journal of Critical Care. 42, 42-46 (2017).
- Barthélémy, R., et al. Accuracy of cumulative volumes of fluid challenge to assess fluid responsiveness in critically ill patients with acute circulatory failure: A pharmacodynamic approach. British Journal of Anaesthesia. 128 (2), 236-243 (2021).
- Ma, G. -. G., et al. Change in left ventricular velocity time integral during Trendelenburg maneuver predicts fluid responsiveness in cardiac surgical patients in the operating room. Quantitative Imaging in Medicine and Surgery. 11 (7), 3133 (2021).
- Monnet, X., et al. Passive leg raising predicts fluid responsiveness in the critically ill. Critical Care Medicine. 34 (5), 1402-1407 (2006).
- Bentzer, P., et al. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids. JAMA. 316 (12), 1298-1309 (2016).
- Monnet, X., Teboul, J. -. L. Passive leg raising. Intensive Care Medicine. 34 (4), 659-663 (2008).
- Kenny, J. -. &. #. 2. 0. 1. ;. S. Functional hemodynamic monitoring with a wireless ultrasound patch. Journal of Cardiothoracic and Vascular Anesthesia. 35 (5), 1509-1515 (2021).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., et al. Inferring the Frank-Starling curve from simultaneous venous and arterial Doppler: Measurements from a wireless, wearable ultrasound patch. Frontiers in Medical Technology. 3, 676995 (2021).
- Sivaciyan, V., Ranganathan, N. Transcutaneous doppler jugular venous flow velocity recording. Circulation. 57 (5), 930-939 (1978).
- Ranganathan, N., Sivaciyan, V., Pryszlak, M., Freeman, M. R. Changes in jugular venous flow velocity after coronary artery bypass grafting. The American Journal of Cardiology. 63 (11), 725-729 (1989).
- Ranganathan, N., Sivaciyan, V. Jugular venous pulse descents patterns - Recognition and clinical relevance. CJC Open. , (2022).
- Abu-Yousef, M. M. Normal and respiratory variations of the hepatic and portal venous duplex Doppler waveforms with simultaneous electrocardiographic correlation. Journal of Ultrasound in Medicine. 11 (6), 263-268 (1992).
- Appleton, C. P., Hatle, L. K., Popp, R. L. Superior vena cava and hepatic vein Doppler echocardiography in healthy adults. Journal of the American College of Cardiology. 10 (5), 1032-1039 (1987).
- Reynolds, T., Appleton, C. P. Doppler flow velocity patterns of the superior vena cava, inferior vena cava, hepatic vein, coronary sinus, and atrial septal defect: A guide for the echocardiographer. Journal of the American Society of Echocardiography. 4 (5), 503-512 (1991).
- Abu-Yousef, M. M., Kakish, M., Mufid, M. Pulsatile venous Doppler flow in lower limbs: Highly indicative of elevated right atrium pressure. American Journal of Roentgenology. 167 (4), 977-980 (1996).
- Iida, N., et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC: Heart Failure. 4 (8), 674-682 (2016).
- Barjaktarevic, I., et al. Ultrasound assessment of the change in carotid corrected flow time in fluid responsiveness in undifferentiated shock. Critical Care Medicine. 46 (11), 1040-1046 (2018).
- Mackenzie, D. C., et al. Ultrasound measurement of carotid flow time changes with volume status. Critical Care. 18 (1), 131 (2014).
- Pace, R., et al. Carotid vs aortic velocity time integral and peak velocity to predict fluid responsiveness in mechanically ventilated patients. A comparative study. Minerva Anestesiologica. 88 (5), 352-360 (2021).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., et al. Carotid artery velocity time integral and corrected flow time measured by a wearable Doppler ultrasound detect stroke volume rise from simulated hemorrhage to transfusion. BMC Research Notes. 15 (1), 7 (2022).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., et al. Carotid Doppler ultrasonography correlates with stroke volume in a human model of hypovolaemia and resuscitation: analysis of 48 570 cardiac cycles. British Journal of Anaesthesia. 127 (2), 60-63 (2021).
- Marik, P. E., Levitov, A., Young, A., Andrews, L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 143 (2), 364-370 (2013).
- Effat, H., Hamed, K., Hamed, G., Mostafa, R., El Hadidy, S. Electrical cardiometry versus carotid Doppler in assessment of fluid responsiveness in critically ill septic patients. Egyptian Journal of Critical Care Medicine. 8 (4), 96-113 (2021).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., et al. A novel, hands-free ultrasound patch for continuous monitoring of quantitative Doppler in the carotid artery. Scientific Reports. 11, 7780 (2021).
- Kenny, J. S., et al. A wireless wearable Doppler ultrasound detects changing stroke volume: Proof-of-principle comparison with trans-esophageal echocardiography during coronary bypass surgery. Bioengineering. 8 (12), 203 (2021).
- Kenny, J. -. E. S., et al. A wearable patch to assess changes in carotid blood velocity during passive leg raising. European Journal of Anesthesiology. 36, 223 (2019).
- Kenny, J. &. #. 2. 0. 1. ;. S., et al. A wearable carotid Doppler tracks changes in the descending aorta and stroke volume induced by end-inspiratory and end-expiratory occlusion: A pilot study. Health Science Reports. 3 (4), 190 (2020).
- Kenny, J. -. E. S., Eibl, J. K., Mackenzie, D. C., Barjaktarevic, I. Guidance of intravenous fluid by ultrasound will improve with technology. Chest. 161 (2), 132-133 (2021).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., Munding, C. E., Eibl, A. M., Eibl, J. K. Wearable ultrasound and provocative hemodynamics: A view of the future. Critical Care. 26 (1), 329 (2022).
- Guarracino, F., et al. Jugular vein distensibility predicts fluid responsiveness in septic patients. Critical Care. 18 (6), 647 (2014).
- Hossein-Nejad, H., Mohammadinejad, P., Ahmadi, F. Internal jugular vein/common carotid artery cross-sectional area ratio and central venous pressure. Journal of Clinical Ultrasound. 44 (5), 312-318 (2016).
- Lipton, B. Estimation of central venous pressure by ultrasound of the internal jugular vein. The American Journal of Emergency Medicine. 18 (4), 432-434 (2000).
- Donahue, S. P., Wood, J. P., Patel, B. M., Quinn, J. V. Correlation of sonographic measurements of the internal jugular vein with central venous pressure. The American Journal of Emergency Medicine. 27 (7), 851-855 (2009).
- Tang, W. W., Kitai, T. Intrarenal venous flow: A window into the congestive kidney failure phenotype of heart failure. JACC: Heart Failure. 4 (8), 683-686 (2016).
- McNaughton, D. A., Abu-Yousef, M. M. Doppler US of the liver made simple. Radiographics. 31 (1), 161-188 (2011).
- Boudoulas, H. Systolic time intervals. European Heart Journal. 11, 93-104 (1990).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., et al. Diagnostic characteristics of 11 formulae for calculating corrected flow time as measured by a wearable Doppler patch. Intensive Care Medicine Experimental. 8 (1), 54 (2020).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., et al. A carotid Doppler patch accurately tracks stroke volume changes during a preload-modifying maneuver in healthy volunteers. Critical Care Explorations. 2 (1), 0072 (2020).
- Kimura, A., Suehiro, K., Juri, T., Tanaka, K., Mori, T. Changes in corrected carotid flow time induced by recruitment maneuver predict fluid responsiveness in patients undergoing general anesthesia. Journal of Clinical Monitoring and Computing. 36 (4), 1069-1077 (2021).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., et al. Carotid Doppler measurement variability in functional hemodynamic monitoring: An analysis of 17,822 cardiac cycles. Critical Care Explorations. 3 (6), 0439 (2021).
- Kenny, J. -. &. #. 2. 0. 1. ;. S., Barjaktarevic, I. Timing and measurement variability are critical when using carotid Doppler to infer hemodynamics. Ultrasound in Medicine and Biology. 46 (12), 3485-3486 (2020).
- Kenny, J., Cannesson, M., Barjaktarevic, I. Minimizing measurement variability in carotid ultrasound evaluations. Journal of Ultrasound in Medicine. 40 (4), 855-856 (2020).
- Lui, E. Y., Steinman, A. H., Cobbold, R. S., Johnston, K. W. Human factors as a source of error in peak Doppler velocity measurement. Journal of Vascular Surgery. 42 (5), 972-979 (2005).
- Gill, R. W. Measurement of blood flow by ultrasound: Accuracy and sources of error. Ultrasound in Medicine and Biology. 11 (4), 625-641 (1985).
- Chebl, R. B., et al. Corrected carotid flow time and passive leg raise as a measure of volume status. American Journal of Emergency Medicine. 37 (8), 1460-1465 (2019).
- Dres, M., et al. Passive leg raising performed before a spontaneous breathing trial predicts weaning-induced cardiac dysfunction. Intensive Care Medicine. 41 (3), 487-494 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved