Method Article
Multi-Photon Laser Ablation of Cytoplasmic Microtubule Organizing Centers in Mouse Oocytes
In This Article
Summary
An optimized protocol is presented that enables the depletion of cytoplasmic microtubule organizing centers in mouse oocytes during metaphase I using a near-infrared femtosecond laser.
Abstract
The fidelity of oocyte meiosis is critical for generating developmentally competent euploid eggs. In mammals, the oocyte undergoes a lengthy arrest at prophase I of the first meiotic division. After puberty and upon meiotic resumption, the nuclear membrane disassembles (nuclear envelope breakdown), and the spindle is assembled mainly at the oocyte center. Initial central spindle positioning is essential to protect against abnormal kinetochore-microtubule (MT) attachments and aneuploidy. The centrally positioned spindle migrates in a time-sensitive manner toward the cortex, and this is a necessary process to extrude a tiny polar body. In mitotic cells, spindle positioning relies on the interaction between centrosome-mediated astral MTs and the cell cortex. On the contrary, mouse oocytes lack classic centrosomes and, instead, contain numerous acentriolar MT organizing centers (MTOCs). At the metaphase I stage, mouse oocytes have two different sets of MTOCs: (1) MTOCs that are clustered and sorted to assemble spindle poles (polar MTOCs), and (2) metaphase cytoplasmic MTOCs (mcMTOCs) that remain in the cytoplasm and do not contribute directly to spindle formation but play a crucial role in regulating spindle positioning and timely spindle migration. Here, a multi-photon laser ablation method is described to selectively deplete endogenously labeled mcMTOCs in oocytes collected from Cep192-eGfp reporter mice. This method contributes to the understanding of the molecular mechanisms underlying spindle positioning and migration in mammalian oocytes.
Introduction
Haploid gametes (sperm and oocyte) are produced through meiosis, which entails one round of DNA replication followed by two consecutive divisions that are necessary for chromosome number reduction prior to fertilization. In mammals, during early fetal life, the oocyte undergoes a lengthy arrest (until puberty) at the diplotene stage of prophase I of the first meiotic division, a stage called the germinal vesicle (GV) stage. Following meiotic resumption, the GV oocyte undergoes nuclear envelope breakdown (NEBD), and the spindle is assembled mainly at the oocyte center1,2,3. Later, driven by F-actin, the spindle migrates in a timely manner from the oocyte center to the cortex to ensure highly asymmetrical division, resulting in an egg with a tiny polar body (PB)4,5,6.
In mitotic cells, the centrosomes consist of a pair of centrioles surrounded by peri-centriolar material components (PMC), such as pericentrin, γ-tubulin, Cep152, and Cep1927. These centriole-containing centrosomes contribute to the fidelity of bipolar spindle formation8. However, centrioles are lost during early oogenesis in various species, including rodents9. Therefore, mouse oocytes adopt a centriole-independent spindle assembly pathway using numerous acentriolar microtubule (MT) organizing centers (MTOCs)9,10. Upon meiotic resumption, the perinuclear MTOCs undergo three distinct steps of recondensation, stretching, and fragmentation into a large number of smaller MTOCs11,12. The fragmented MTOCs are then clustered and sorted to organize a bipolar spindle10,13,14. Another pool of MTOCs is located in the cytoplasm during NEBD. Some of these cytoplasmic MTOCs migrate and form spindle poles (polar MTOCs, pMTOCs)10,11. Recently, another subset of cytoplasmic MTOCs was discovered, termed metaphase cytoplasmic MTOCs (mcMTOCs), that do not contribute to spindle pole formation but remain in the oocyte cytoplasm during metaphase I (Met I)15. Depleting mcMTOCs by multi-photon laser ablation or abnormally increasing their numbers by autophagy inhibition perturbs spindle positioning and migration and increases the incidence of aneuploidy in metaphase II oocytes15.
Interestingly, mcMTOCs differ from pMTOCs in many aspects15. For example, in contrast to pMTOCs, which mainly originate from perinuclear MTOCs, mcMTOCs originate from the oocyte cortex. When the spindle is still at the oocyte center, the mcMTOCs are localized asymmetrically opposite to the side to which the spindle migrates for PB extrusion15. Astral-like MTs cannot reach the cortex in the relatively large oocyte cell. Therefore, these mcMTOCs nucleate MTs to anchor the spindle (via astral-like MTOCs) to the cortex. These findings suggest a model in which the mcMTOC-nucleated MT force counteracts the F-actin-mediated force that drives spindle migration toward the cortex. The balance between these two opposing forces is essential to regulate the central spindle positioning and timely spindle migration15.
To date, all the examined PMC proteins (pericentrin, g-tubulin, Cep192, and Aurora kinase A) localize to both MTOC pools: mcMTOCs and pMTOCs15. Therefore, there is no chemical or genetic approach to selectively perturb mcMTOCs without perturbing pMTOCs. These limitations can be circumvented by selectively targeting the mcMTOCs with laser ablation. Among the laser-based technologies developed for microablation, pulsed multi-photon femtosecond lasers show great potential due to their precision impact limited to the focal plane, the high penetration depth of near-infrared light, and the reduced phototoxicity and thermal damage to the cell16,17,18. This work describes a selective approach to ablate mcMTOCs in mouse oocytes using a multi-photon laser coupled to an inverted microscope.
Protocol
All the methods described here were approved by the University of Missouri (Animal Care Quality Assurance Ref. Number 9695). Cep192-eGFP reporter female mice aged 6-8 weeks old were used in the present study. To generate Cep192-eGFP reporter mice, CRISPR/Cas9-mediated homology-directed repair was used to integrate the EGFP reporter gene into the CF-1 mouse genome. The EGFP reporter was fused at the C-terminus of Cep192 (an integral component of MTOCs)15. To maintain the mouse colony, homozygous Cep192-eGfp reporter mice were used. All the animals were maintained in cages (up to four animals/cage) at 21 °C and 55% humidity, with a 12 h light/dark cycle and ad libitum access to food and water.
1. Mouse oocyte collection
- Prepare the culture medium (Chatot, Ziomek, and Bavister, CZB19, see the Table of Materials), and incubate it at 37 °C and 5% CO2 overnight.
NOTE: The CZB medium can be stored at 4 °C for 1 month (see Supplementary File 1 for the media composition). - Supplement the CZB medium with glutamine (1 mM) and milrinone (2.5 mM) (CZB + M) (see Table of Materials), and place it in the incubator.
NOTE: Milrinone is a phosphodiesterase inhibitor that maintains the oocytes arrested at prophase I and prevents meiotic resumption20. - Prepare the collection medium (bicarbonate-free minimal essential medium, MEM) containing 3 mg/mL polyvinylpyrolidone (PVP), 25 mM HEPES (pH 7.3) (Supplementary File 1), and milrinone (2.5 mM) (MEM/PVP + M, see the Table of Materials).
- Prepare the collection and culture dishes, make four microdrops (100 µL) of collection medium (MEM/PVP + M) and two microdrops (100 µL) of culture (CZB + M) medium in 60 mm and 35 mm Petri dishes, respectively, and cover them with mineral oil (see Table of Materials). Keep the collection dish on the slide warmer, and put the culture dish into the incubator at 37 °C and 5% CO2.
- Inject intraperitoneally 5 IU of pregnant mare's serum gonadotropin (PMSG, see the Table of Materials) into sexually mature (6-8 week-old) Cep192-eGFP reporter female mice 44-48 h before oocyte collection.
- Sacrifice the mice by cervical dislocation, identify and remove the ovaries21, and transfer them into a watch glass containing prewarmed collection medium (MEM/PVP + M) at 37 °C.
- Fix the ovary by touching a 1 mL syringe to the bottom of the watch glass and puncturing it several times (~40 times per ovary) using sewing needles bundled together to release the oocytes into the medium.
- Using a plastic transfer pipet, transfer all the medium containing the cumulus-oocyte complexes (COCs) from step 1.7 into an empty 100 mm plastic Petri dish.
- Under a stereomicroscope, collect the COCs using a Pasteur glass pipet, and transfer them to the collection dish that contains MEM/PVP + M.
- Using a narrow Pasteur glass pipet (approximately 100 µm in diameter), denude the oocytes mechanically by gentle repetitive pipetting, followed by transferring and washing them in four microdrops of (100 µL) MEM/PVP + M (the collection dish) prior to their transfer into the CZB + M culture dish.
- Incubate the denuded oocytes for 1 h at 37 °C with 5% of CO2 in the air.
2. Oocyte microinjection
- Place a 250 µL drop of MEM/PVP + M into a 100 mm plastic Petri dish lid, and cover it with mineral oil.
NOTE: The Petri dish lid has a lower rim that provides more space for adjusting the micromanipulator. - Turn on the microinjection system (see the Table of Materials).
- Place the microinjection dish on the warming stage (37 °C) of the microscope.
- Load the injection needle with 0.5 µL of mCh-Cep192cRNA using microloader pipette tips (see Table of Materials), and secure the injection needle to the micromanipulator.
- Transfer the denuded oocytes (step 1.11) to the 250 µL microdrop (step 2.1).
- Under a 20x or 40x objective lens, adjust the position and the focus of the injection and holding needles according to the oocyte position (Figure 1).
- Set up the microinjection unit, and set the injection pressure (pi), compensation pressure (pc), and injection time (ti) to be able to inject 5-10 pl of mCh-Cep192 cRNA (to exogenously label the MTOCs).
- Carefully inject the oocytes without touching the nucleus.
- Once all the oocytes are injected, wash them in three microdrops of CZB + M, transfer them to the culture dish (CZB + M), and incubate them for 3 h at 37 °C to allow mCh-Cep192 expression.
3. Oocyte maturation
- Prepare the maturation medium by adding glutamine (1 mM) to CZB medium pre-equilibrated in an incubator with 5% CO2 in air at 37 °C for at least 3 h.
- Make two microdrops (100 µL) of the maturation medium in a 35 mm Petri dish, and cover them with mineral oil (maturation dish).
- Wash prophase I-arrested oocytes at least three times in 100 µL milrinone-free CZB microdrops to completely remove the milrinone and allow meiotic resumption.
- Transfer the oocytes to the maturation dish, and incubate them for 5 h (prometaphase I stage) in a humidified incubator with 5% CO2 in air at 37 °C.
4. Oocyte preparation for ablation and imaging
- Transfer the prometaphase I oocytes (step 3.4) to a glass-bottom culture dish containing 100 µL of maturation medium (step 3.1) covered with mineral oil.
5. Microscope preparation for ablation
- At least 30 min before ablation, turn on the temperature controller for the stage incubator (see Table of Materials) and set it at 37 °C.
- Turn on the CO2 controller and set it at 5% CO2.
- Select a 40x oil immersion apochromatic objective, and apply a small drop of the immersion oil.
- Mount the glass-bottom culture dish with the oocytes on a stage-top incubator, and cover the incubator with a gas lid.
- In the image acquisition software (see the Table of Materials), select an option enabling the saving of multiple stage positions (e.g., "Define mark and find experiment"). Using transmitted light brightfield illumination, center on the individual oocytes and save their positions.
- In the image acquisition software, select the XYZ scanning mode, set the image format to 256 x 256 pixels, set the zoom factor to 2.5x - 3.0x, and select a scanning frequency of 600 Hz. This corresponds to a pixel dwell time of approximately 1.6 µs.
- Set a 488 nm excitation laser line at approximately 10% of the laser power (which corresponds to 4.0 µW at the sample level) (see Table of Materials), and use a spectral bandwidth of 500-550 nm to observe the GFP signal from the MTOCs. For the simultaneous observation of fluorescence from the mCherry-tagged Cep192, set a 585 nm excitation laser line at approximately 8% of the laser power (which corresponds to 10.7 µW at the sample level), and use another detector set to a spectral bandwidth of 595-645 nm.
NOTE: It is helpful to use the confocal microscope's transmitted light detector (TLD) for concurrently detecting oocyte boundaries. - Using a live scanning mode and the manual control of the z-drive, screen the oocyte for MTOCs.
- Once an mcMTOC is detected, draw a square region of interest (ROI) around it.
6. Ablation of mcMTOCs
- Set a femtosecond laser to a 740 nm wavelength.
NOTE: The laser wavelength can be changed according to the microscope and the laser conditions. - Use the electro-optic modulator of the multi-photon microscope (see Table of Materials) to set the laser power to 70%-80%, corresponding to 60-70 mW power at the sample plane.
NOTE: If the laser is equipped with a femtosecond pulse compensator, use it to correct for group delay dispersion (GDD). GDD correction reduces the amount of laser power required for efficient mcMTOC ablation and minimizes photodamage to the oocyte. The GDD control is usually integrated with the image acquisition software of commercial multi-photon microscopes. - Use the same image format, zoom factor, and scanning frequency as in step 5.6. Set the line and frame averaging parameters to 1.
- Click on the Scan button in the software to perform the ablation of the mcMTOC by conducting a single laser scan of the selected ROI.
- Use the channel settings from step 5.7 to review the results of the ablation by comparing the images of the GFP-labeled structures taken before and after the multi-photon laser exposure. If the ablation is successful, the intensity of the GFP fluorescence in the targeted mcMTOC decreases to the levels observed in the background.
- If some fragments of a GFP-labeling structure remain, repeat step 6.4 one or more times by focusing on various z planes of the mcMTOC.
- Use the channel settings from step 5.7 to verify the ablation efficiency by the complete loss of mcMTOC-associated mCherry signals (mCh-Cep192).
- Repeat step 5.8 to step 6.7 until all the MTOCs of an oocyte (at various focal planes) are ablated.
NOTE: This protocol uses mCh-Cep192 microinjection as a strategy to confirm mcMTOC depletion following multi-photon laser exposure and exclude the possibility that the loss of GFP fluorescence is caused by GFP photobleaching. However, the microinjection of this probe is optional and not required for the ablation procedure.
Results
Multi-photon laser ablation provides an efficient method to selectively ablate intracellular structures. The current study employed multi-photon laser ablation to deplete mcMTOCs in mouse oocytes selectively. Laser ablation depleted the mcMTOCs efficiently, as shown by the reduction in the endogenous GFP fluorescence in the targeted mcMTOCs to a level comparable to the background. Exogenous mCh-Cep192 in the targeted mcMTOCs was also abolished following laser ablation. The laser ablation of mcMTOCs should be performed at different focal planes within the oocyte (where mcMTOCs are located, Figure 2) to ensure that all the mcMTOCs within the oocyte are depleted (Figure 3).
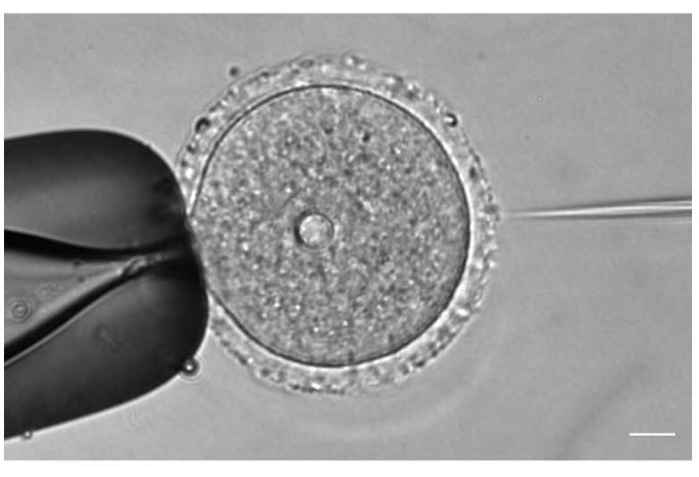
Figure 1: Oocyte microinjection. Microinjection of a prophase I-arrested mouse oocyte with mCh-Cep192 cRNA. Scale bar: 10 µm. Please click here to view a larger version of this figure.
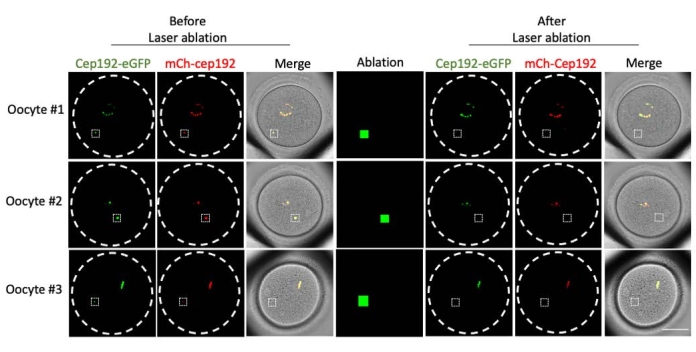
Figure 2: Depletion of mcMTOCs in mouse oocytes. Representative images of single focal planes. The white squares indicate an mcMTOC before and after multi-photon laser ablation. Scale bar: 40 µm. Please click here to view a larger version of this figure.
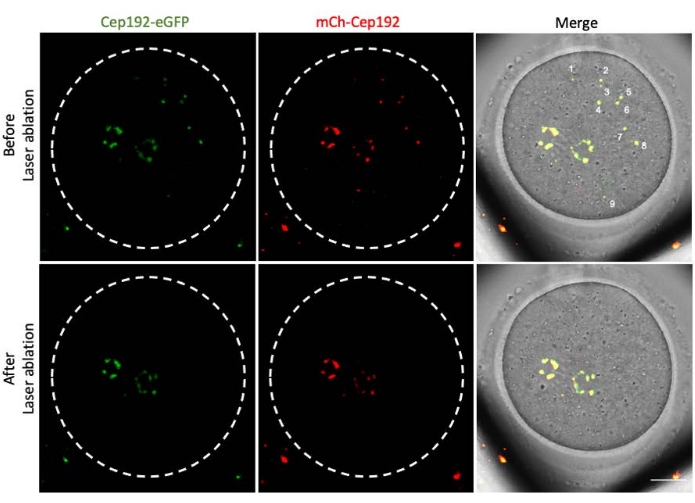
Figure 3: Depletion of mcMTOCs at different focal planes in a mouse oocyte. Representative maximum projection images of an mcMTOC-ablated mouse oocyte. Scale bar: 20 µm. Please click here to view a larger version of this figure.
Supplementary File 1: Compositions of Chatot, Ziomek, and Bavister (CZB) and bicarbonate-free minimal essential medium (MEM/PVP). Please click here to download this File.
Discussion
Different methods exist to disturb the cytoskeleton-related structures within cells22,23,24,25. However, finding efficient techniques to selectively perturb the targeted structure without compromising the cell viability is challenging. The multi-photon laser ablation method presented here is an efficient strategy to induce a selective mechanical perturbation to mcMTOCs within the oocyte without altering the oocyte viability.
Laser ablation has been extensively used to understand the molecular mechanisms controlling chromosome segregation during mitosis and meiosis22,23,26,27. Due to the relatively large size (>80 mm in diameter) of mammalian oocytes compared to somatic cells28, the ablation of their intracellular structures represents a challenge. Moreover, the average mcMTOC volume in metaphase I oocytes is ~20 µm15, representing an additional challenge. An efficient method that offers deeper tissue penetration must be adopted to overcome these challenges. The main advantage of using the multi-photon laser for ablation is its ability to reach deeper into the cell while minimizing off-target effects29.
To verify the efficacy and efficiency of the laser ablation method to deplete the targeted structure, it is recommended to use a fluorescently labeled protein to identify the targeted structure over time (before and after ablation)23. It is important to notice that laser ablation depletes the whole mcMTOC as a structure, and although smaller mcMTOCs require only a single laser exposure to be depleted, larger mcMTOCs may require more than one laser exposure at different focal planes. It is also recommended to fix and immunolabel a subset of control and mcMTOC-ablated oocytes with an MTOC marker (such as γ-tubulin, pericentrin, or Cep192) to confirm the efficiency of the mcMTOC ablation further. In control oocytes, areas of the cytoplasm just adjacent to but not overlapping with mcMTOCs will be exposed to the laser.
This experiment requires several mcMTOC ablations while moving among different focal planes within the oocytes. Therefore, it is highly recommended to practice this technique several times before executing the experiment to minimize the experiment time, thereby increasing oocyte viability. Moreover, it is important to use the minimum laser power that is sufficient to deplete the mcMTOCs without affecting oocyte viability.
This technique has some limitations. First, multi-photon confocal microscopes are relatively more expensive than regular confocal microscopes. Second, perturbing all mcMTOCs at different focal planes is more time-consuming than chemical or genetic perturbations. Third, this protocol requires technical skills to ablate all mcMTOCs in the shortest possible time. However, once mastered, the use of the multi-photon laser provides an excellent strategy to perturb several intracellular structures in mouse oocytes, including mcMTOCs, contributing to the understanding of molecular mechanisms regulating spindle positioning and its timely migration in mammalian oocytes.
Disclosures
The authors declare no competing interests.
Acknowledgements
The authors would like to thank all members of the Balboula laboratory for their valuable help and discussions. The authors thank Melina Schuh for kindly sharing the mCherry-Cep192 construct. This study was supported by R35GM142537 (NIGMS, NIH) to AZB.
Materials
| Name | Company | Catalog Number | Comments |
| 4 IN thinwall GL 1.0 OD/.75 ID | World precision instrument | TW100F-4 | Injection needles |
| Borosilicate glass | Fisherbrand | Cat# 13-678-20D | |
| Borosilicate glass capillarities | World Precision Instrument | Cat# TW100-6 | Holding needles |
| Bovine serum albumine | MilliporeSigma | Cat# A4503 | |
| Cage incubator for Leica DMI6000 B microscope | Life Imaging Services GmbH | ||
| Calcium chlrode dihydrate | MilliporeSigma | Cat# C7902 | |
| CO2 controller | Pecon | # 0506.000 | |
| CO2 Cover HP | Pecon | # 0506.020 | |
| DL-Lactic acid | MilliporeSigma | Cat# L7900 | |
| DMi8 | Leica | N/A | Microscope |
| EDTA | MilliporeSigma | Cat# E5134 | |
| Femtojet 4i | Eppendorf | N/A | Microinjector |
| Femtotips Microloader | Fisher scientific | E5242956003 | |
| Gentamicin | MilliporeSigma | Cat# G1272 | |
| Gentamycin | MilliporeSigma | Cat# 1272 | |
| Glass bottom dish | Mat Tek Corporation | Cat# P35G-1.0-20-C | |
| Hepes | MilliporeSigma | Cat# H3784 | |
| Hepes Sodium Salt | MilliporeSigma | Cat # H3784 | |
| Hera Cell vios 160i | Thermo | N/A | CO2 incubator |
| Leica TCP SP8 spectral laser scanning confocal micorscope with inverted stand DMI6000 B | Leica Microsystems, Inc | N/A | |
| L-Glutamine | MilliporeSigma | Cat#G8540 | |
| Magnesium sulfate dihydrate | MilliporeSigma | Cat# M7774 | |
| MaiTai DeepSee Ti-Sapphire femtosecond laser | Spectra-Physics | N/A | |
| mCH-Cep192 cRNA | N/A | ||
| Medium Essential Medium Eagle - MEM | MilliporeSigma | Cat #M0258 | |
| Milrinone | MilliporeSigma | Cat# M4659 | |
| Mineral oil | MilliporeSigma | Cat# M5310 | |
| Minimum essential medium eagle (MEM) | MilliporeSigma | Cat# M0268 - 1L | |
| Mouse: Cep192-eGFP reporter CF-1 | N/A | ||
| Petri dish (100 mm) | Fisherbrand | Cat# FB0875712 | |
| Petri dish (35 mm) | Corning | Cat# 430165 | |
| Petri dish (60 mm) | Falcon | Cat# 351007 | |
| Phenol Red | MilliporeSigma | Cat# P5530 | |
| Polyvinylpyrolidone | MilliporeSigma | Cat# P2307 | |
| Polyvinylpyrolidone (PVP) | MilliporeSigma | Cat# P2307 | |
| Potassium chloride | MilliporeSigma | Cat# P5405 | |
| Potassium phosphate monobasic | MilliporeSigma | Cat# P5655 | |
| Pregnant mare´s serum gonadotropin | Lee BioSolutions | Cat# 493-10-10 | |
| Pyruvic acid | MilliporeSigma | Cat# P4562 | |
| Pyruvic acid | MilliporeSigma | Cat# P4562 | |
| Sewing needles | D.M.C | N/A | N° 5 * 16 Needles |
| Sodium bicarbonate | MilliporeSigma | Cat# S5761 | |
| Sodium chloride | MilliporeSigma | Cat# S5886 | |
| Stage-top heating insert P | Pecon | # 0426.300 | |
| Sterezoom S9i | Leica | N/A | Stereomicroscope |
| Syringe 1 mL | BD company | Cat# 309597 | |
| Taurine | MilliporeSigma | Cat# T0625 | |
| Temperature controller Tempcontrol 37-2 digital | Pecon | # 0503.000 | |
| The Cube temperature controller for the cage incubator | Life Imaging Services GmbH |
References
- Bennabi, I., Terret, M. E., Verlhac, M. H. Meiotic spindle assembly and chromosome segregation in oocytes. Journal of Cell Biology. 215 (5), 611-619 (2016).
- Hashimoto, N., Kishimoto, T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Development Biology. 126 (2), 242-252 (1988).
- Kitajima, T. S., Ohsugi, M., Ellenberg, J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 146 (4), 568-581 (2011).
- Azoury, J., Verlhac, M. H., Dumont, J. Actin filaments: Key players in the control of asymmetric divisions in mouse oocytes. Biology of the Cell. 101 (2), 69-76 (2009).
- Li, H., Guo, F., Rubinstein, B., Li, R. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nature Cell Biology. 10 (11), 1301-1308 (2008).
- Schuh, M., Ellenberg, J. A new model for asymmetric spindle positioning in mouse oocytes. Current Biology. 18 (24), 1986-1992 (2008).
- Pimenta-Marques, A., Bettencourt-Dias, M. Pericentriolar material. Current Biology. 30 (12), 687-689 (2020).
- Hinchcliffe, E. H. The centrosome and bipolar spindle assembly: Does one have anything to do with the other. Cell Cycle. 10 (22), 3841-3848 (2011).
- Szollosi, D., Calarco, P., Donahue, R. P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. Journal of Cell Science. 11 (2), 521-541 (1972).
- Schuh, M., Ellenberg, J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 130 (3), 484-498 (2007).
- Clift, D., Schuh, M. A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nature Communications. 6, 7217 (2015).
- Luksza, M., Queguigner, I., Verlhac, M. H., Brunet, S. Rebuilding MTOCs upon centriole loss during mouse oogenesis. Developmental Biology. 382 (1), 48-56 (2013).
- Balboula, A. Z., et al. Haspin kinase regulates microtubule-organizing center clustering and stability through Aurora kinase C in mouse oocytes. Journal of Cell Science. 129 (19), 3648-3660 (2016).
- Breuer, M., et al. HURP permits MTOC sorting for robust meiotic spindle bipolarity, similar to extra centrosome clustering in cancer cells. Journal of Cell Biology. 191 (7), 1251-1260 (2010).
- Londoño-Vásquez, D., Rodriguez-Lukey, K., Behura, S. K., Balboula, A. Z. Microtubule organizing centers regulate spindle positioning in mouse oocytes. Developmental Cell. 57 (2), 197-211 (2022).
- Konig, K., Riemann, I., Fischer, P., Halbhuber, K. J. Intracellular nanosurgery with near infrared femtosecond laser pulses. Cellular and Molecular Biology. 45 (2), 195-201 (1999).
- Galbraith, J. A., Terasaki, M. Controlled damage in thick specimens by multiphoton excitation. Molecular Biology of the Cell. 14 (5), 1808-1817 (2003).
- Maghelli, N., Tolic-Norrelykke, I. M. Laser ablation of the microtubule cytoskeleton: Setting up and working with an ablation system. Methods in Molecular Biology. 777, 261-271 (2011).
- Chatot, C. L., Ziomek, C. A., Bavister, B. D., Lewis, J. L., Torres, I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. Journal of Reproduction and Fertility. 86 (2), 679-688 (1989).
- Tsafriri, A., Chun, S. Y., Zhang, R., Hsueh, A. J., Conti, M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: Studies using selective phosphodiesterase inhibitors. Developmental Biology. 178 (2), 393-402 (1996).
- Greaney, J., Subramanian, G. N., Ye, Y., Homer, H. Isolation and in vitro culture of mouse oocytes. Bio Protocol. 11 (15), 4104 (2021).
- Khodjakov, A., Cole, R. W., Rieder, C. L. A synergy of technologies: Combining laser microsurgery with green fluorescent protein tagging. Cell Motility and the Cytoskeleton. 38 (4), 311-317 (1997).
- Pavin, N., Tolic, I. M. Mechanobiology of the mitotic spindle. Developmental Cell. 56 (2), 192-201 (2021).
- Khodjakov, A., Cole, R. W., Oakley, B. R., Rieder, C. L. Centrosome-independent mitotic spindle formation in vertebrates. Current Biology. 10 (2), 59-67 (2000).
- Aist, J. R., Liang, H., Berns, M. W. Astral and spindle forces in PtK2 cells during anaphase B: a laser microbeam study. Journal of Cell Science. 104, 1207-1216 (1993).
- Bennabi, I., Manil-Segalen, M. Laser Ablation of microtubule-chromosome attachment in mouse oocytes. Methods in Molecular Biology. 1818, 153-161 (2018).
- Milas, A., Jagric, M., Martincic, J., Tolic, I. M. Optogenetic reversible knocksideways, laser ablation, and photoactivation on the mitotic spindle in human cells. Methods in Cell Biology. 145, 191-215 (2018).
- Warzych, E., Lipinska, P. Energy metabolism of follicular environment during oocyte growth and maturation. Journal of Reproduction and Development. 66 (1), 1-7 (2020).
- Denk, W., Strickler, J. H., Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science. 248 (4951), 73-76 (1990).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved