Method Article
Endoscopic Bilateral Nipple-sparing Mastectomy via a Single Axillary Incision with Immediate Pre-pectoral Implant-based Breast Reconstruction
In This Article
Summary
Here, we present detailed surgical procedures for endoscopic bilateral nipple-sparing mastectomy via a single axillary incision with immediate pre-pectoral implant-based breast reconstruction. This procedure is a secure and viable method for breast cancer patients.
Abstract
Oncoplastic breast surgery, with its focus on improving cosmetic outcomes while maintaining oncological safety, has fundamentally transformed the landscape of breast cancer surgical treatment, giving rise to an array of techniques for breast reconstruction. Nipple-sparing mastectomy (NSM) with immediate implant-based breast reconstruction (IBBR) has emerged as a cornerstone in managing early breast cancer. Aligned with the principles of minimally invasive surgery, recent years have witnessed the widespread integration of endoscopic approaches in breast surgery, encompassing procedures like endoscopic breast-conserving surgery (E-BCS) and endoscopic nipple-sparing mastectomy (E-NSM), among others. Capitalizing on the advantages of inconspicuous and shorter incisions, improved visibility, and the avoidance of radiation therapy, the popularity of E-NSM with IBBR is on the rise. However, conventional E-NSM with IBBR often requires two or more incisions, which can result in suboptimal cosmetic outcomes and even prosthesis loss.This paper presents a comprehensive account of the intricate surgical procedures involved in endoscopic bilateral nipple-sparing mastectomy with immediate pre-pectoral implant-based breast reconstruction. The insights shared are drawn from the collective experience of our institution. Notable benefits associated with the described surgical approach encompass enhanced cosmetic outcomes, improved postoperative quality of life, and enhanced physiological functions attributable to the application of pre-pectoral implant-based breast reconstruction through a single incision.
Introduction
As breast cancer treatment continues to advance, an increasing number of breast surgeons are shifting their focus beyond solely addressing malignant tumors. They also place greater emphasis on addressing the post-treatment appearance and quality of life of their patients. Nipple-sparing mastectomy (NSM), characterized by comparable oncologic outcomes, favorable patient satisfaction, and cosmetic outcomes, has now become a standard surgical option for patients with bilateral early breast cancer and those undergoing prophylactic mastectomy1,2,3.
Endoscopic breast surgery (EBS) is an emerging and forward-looking surgical approach that is gaining popularity in both nipple-sparing mastectomy (NSM) and breast-conserving surgery. When compared to traditional breast surgery methods, EBS has demonstrated superior cosmetic outcomes, increased patient satisfaction owing to its less noticeable incisions, and similar levels of oncological safety4,5,6.
However, the conventional approach to endoscopic nipple-sparing mastectomy (E-NSM) with immediate implant-based breast reconstruction (IBBR) often requires two or more incisions, typically located in the axilla, anterior axillary line, and peri-areolar regions4,7. Unfortunately, this technique frequently yields suboptimal cosmetic outcomes. This is especially pronounced in cases involving peri-areolar incisions, where E-NSM eliminates the vertical blood supply route to the nipple-areola complex (NAC), relying solely on the dermal vascular network for nourishment8. This scenario potentially leads to ischemic necrosis in the NAC. Moreover, for pre-pectoral breast reconstruction, incisions on the breast surface can create openings for infection or flap ischemia, increasing the risk of prosthetic exposure, nipple necrosis, and even prosthesis loss7,9,10. Given the rapid advancements in endoscopic techniques and instrumentation, novel variations of E-NSM with IBBR predominantly focus on a single axillary incision and insufflation technique11,12. These innovations offer distinct advantages, including reduced risk of nipple necrosis, decreased patient discomfort leading to quicker recovery, significantly improved cosmetic outcomes, and optimized patient benefits, among others12,13.
This article provides a comprehensive description of the surgical procedures involved in endoscopic bilateral nipple-sparing mastectomy via a single axillary incision with immediate pre-pectoral implant-based breast reconstruction. The aim is to showcase the feasibility, exceptional cosmetic outcomes, and physiological considerations associated with this surgical approach.
To facilitate readers' comprehension of the protocol, we present a representative case for elucidation. The patient was a 33-year-old female who exhibited bilateral breast lumps on ultrasound, categorized as BI-RADS 4, with relatively large-sized lumps (Figure 1). Histopathological confirmation through Mammotome-assisted minimally invasive resection identified bilateral ductal carcinoma in situ14 (Figure 2). This young woman had bilateral nipple asymmetry and a strong desire to rectify it. Given her passion for dance, she also had a significant demand for favorable cosmetic outcomes. After introducing our surgical plan, the patient ultimately opted to undergo endoscopic bilateral nipple-sparing mastectomy via a single axillary incision with immediate pre-pectoral implant-based breast reconstruction.
Protocol
The study was approved by the Ethics Review Committee of The Sixth Affiliated Hospital, Sun Yat-sen University (No.2023ZSLYEC-171). Photographs and videos were utilized with informed consent duly obtained from the patients. The video is for educational purposes only.
1. Preoperative marking
- Mark the inferior fold of the axilla, the edge of the breast, and the inframammary fold of bilateral breasts (Figure 3).
2. Positioning and anesthesia
- Induce general anesthesia and perform tracheal intubation.
- Place the patient in a supine position with bilateral arms abducted at 90°.
- Sterilize the surgical area with povidone-iodine skin disinfectant.
- Wrap the bilateral limbs with sterile drapes and fix them abducted at 90°.
3. Sentinel lymph node biopsy (SLNB)
- Use a 1 mL syringe to aspirate a suspension mixture of carbon nanoparticles in saline, mixed in a 1:1 ratio to achieve a total volume of 1 mL, for the formulation of the sentinel lymph node tracer. Inject the tracer (0.3 mL) into the parenchyma of the outer upper quadrant of the breast at any 3 points, massage for 5 min, and wait for 5 min.
- Use a 10 mL syringe to aspirate a mixture of methylene blue and saline in a 1:5 ratio to a total volume of 10 mL and inject it into the circum-mammary ligaments of the breast, known as the breast footprint15, guided by ultrasound or preoperative marking to make the boundary and extent of dissection.
NOTE: Staining is not a necessary step in the surgery. It assists breast surgeons who have not yet overcome the learning curve to control the extent of dissection. - Make an approximately 4 cm curved axillary incision along the line marked previously to complete SLNB under direct vision.
NOTE: The sentinel lymph node biopsy was examined to be negative by an intraoperative frozen section biopsy.- Perform a complete axillary lymph node dissection (ALND) with the removal of level I and II axillary lymph nodes if SLNB is positive.
- Ensure that the anterior edge of the incision is more than 1 cm from the anterior edge of the pectoralis major muscle and does not extend beyond the anterior axillary line. If necessary, the incision can be appropriately extended posteriorly.
4. Air cavity building
- Bend the arm of the patient on the operation side and secure it to the head rack.
- Seek the lateral edge of the pectoralis major muscle from the axillary incision. Dissect the retro-mammary space for 2 cm and the subcutaneous flap for 3 cm under direct vision.
- Insert the wound protector into the incision, then connect with the four-trocar single port (Figure 4). Place a 12 mm trocar above, 10 mm trocar below, and two 5 mm trocars on both sides.
- Connect the 12 mm trocar with the constant pressure pneumoperitoneal device to construct a working space. Maintain the air cavity by applying CO2 insufflation at 8 mmHg pressure and 40 L/min air flow.
- Then insert an oblique-ended rigid endoscope measuring 5 mm in diameter with a viewing angle of 30° through the 10 mm trocar.
- Insert an electric hook and laparoscopic grasping forceps through two 5 mm trocars, respectively.
5. Retromammary space dissection
- Use the circum-mammary ligaments as the boundary of the breast, paying attention to exposing and identifying ligamentous structures15. The integrity of the ligament can be ensured by staining, finger-press, and resistance from endoscopic instruments.
NOTE: The assistant can help with finger pressing during the operation. Additionally, the chief surgeon can temporarily suspend the endoscopic operation and do finger pressing for assessment. None of these procedures impacts observation under endoscopic vision. - Perform the dissection by following the sequence shown in Figure 5. Dissect the retro-mammary space under endoscopic vision using the electric hook. Extend this dissection superiorly to the subclavian ligament, medially to the parasternal ligament, inferiorly to the inframammary fold, and laterally to the lateral border of the mammary gland.
NOTE: In this case, ductal carcinoma was confirmed preoperatively (see Figure 2), therefore, the pectoralis major fascia is completely preserved intraoperatively16,17. This approach serves a dual purpose: it minimizes surgical trauma and postoperative discomfort, thereby improving the patient's quality of life. Additionally, preserving the pectoralis major fascia allows for secure mesh fixation, preventing prosthesis displacement.
6. Subcutaneous layer dissection
- Pull the wound protector to reach the subcutaneous layer.
NOTE: The following measures can ensure that surgeons have reached the subcutaneous layer. Firstly, during the air cavity-building phase, a portion of the subcutaneous flap is dissected under direct vision. Secondly, through the magnifying effect of the endoscope, the superficial fascia of the breast can be clearly observed under endoscopic vision. Thirdly, the breast is suspended on the dermal layer through Cooper's ligaments, which can be observed under the endoscope, guiding surgeons into the dissection of the subcutaneous layer18. - Follow the zoning dissection sequence (Figure 6) and perform the dissection with the electric hook in the following sequence: outer upper quadrant, inner upper quadrant, outer lower quadrant, retro nipple area, and inner lower quadrant.
- Use endoscopic scissors to cut off the root of the nipple, preventing NAC necrosis caused by thermal effects.
- Take a tissue biopsy specimen from the posterior margin of the nipple for intraoperative frozen sectioning.
- Utilize the electric hook to carefully dissociate the inner lower quadrant of the mammary gland.
- Remove the intact breast specimen through the axillary incision.
NOTE: Achieving hemostasis through a single port under endoscopic vision is difficult. Using the electric hook to achieve hemostasis may lead to eschar formation at the bleeding site, potentially hindering the observation of the bleeding point and posing a risk of skin burns.
7. Implant placement
- Weigh the breast specimen, measure the diameter of the implant cavity, and combine the preoperative measurements to select the appropriate prosthesis.
- Irrigate the implant cavity with 2,000 mL of warm, sterile distilled water.
- Adjust the implant cavity pressure to 5 mmHg to carefully inspect the implant cavity and complete hemostasis under endoscopic vision.
- Soak the implant cavity with 0.45%-0.55% povidone-iodine solution for 10 min, disinfect the surgical region, and change gloves.
- Cover the prosthesis fully with 2 meshes. Suture the meshes with 3-0 absorbable sutures and simple interrupted sutures for fixation.
- Insert the prosthesis into the implant cavity.
8. Drainage
- Place one drainage tube on each of the inframammary fold and axilla in the subcutaneous layer, maintained under low negative pressure suction.
- Close the axillary incision wound with 4-0 absorbable sutures (Figure 7).
9. Postoperative nursing
- Apply an elastic bandage to achieve pressure dressing of the breast and axilla.
- Perform proper pressure dressing at the axillary wound.
NOTE: It is mainly pressurized to the superior and inferior margins of the prosthesis rather than the nipple-areolar complex in order to prevent ischemic necrosis of the NAC. - Administer proper prophylactic antibiotics for 24 h (1.5 g of cefuroxime dissolved in 100 mL of saline) administered intravenously.
- Pay attention to the blood circulation of the NAC when changing the wound dressing. Apply nitroglycerin around the NAC to promote blood circulation if necessary. Look at the color of the NAC, the presence of edema, and bruising.
- Maintain the patency of the drainage tubes; observe and record the color, nature, and drainage volume of the drainage fluid daily. If the drainage volume is less than 20 mL for 24 h for 3 days, remove the drainage tubes.
- Ask the patient to perform functional exercises of the upper limbs for 2 weeks postoperatively19.
- Ensure the patient wears a pressure garment for 3-6 months starting on the 2nd postoperative day.
- Ensure the patient performs breast implant displacement exercises for smooth implants20,21,22.
NOTE: The contralateral breast is operated in the same way.
Results
The surgical procedure lasted for 4 h, with a recorded blood loss of 30 mL. Postoperative recovery proceeded without significant complications. Drainage tubes were removed on the 7th day post-surgery, and the patient was discharged on the 10th day (Figure 8). Subsequent to postoperative pathological analysis, ductal carcinoma in situ was confirmed in the residual mammary gland on the left side. At the same time, no cancer cells were observed in the right residual mammary gland. Final pathologic evaluations of both bilateral nipple bases and sentinel lymph nodes yielded negative results for malignancy. At the five-month mark after the operation, the patient expressed satisfaction with her breast's appearance (Figure 9).
Between June 2021 and October 2022, a cohort of 10 patients diagnosed with bilateral breast cancer (BBC) or necessitating contralateral prophylactic mastectomy (CPM) underwent endoscopic bilateral nipple-sparing mastectomy via a single axillary incision with immediate prepectoral implant-based breast reconstruction. The average age of the patients was 42 years, with an average body mass index (BMI) of 22.12 Kg/m2. The mean duration of the surgical procedure was 4.25 h, and the average volume of intraoperative blood loss measured 33 mL. The postoperative recovery of each patient was characterized by an uneventful course devoid of significant complications, and the typical duration of postoperative hospitalization averaged 11 days. Subsequently, all patients received regular follow-up care, with appointments scheduled every three months throughout the initial two years following surgery (Table 1). Notably, the Harris scores of three patients were rated as excellent, while seven patients received a rating of good 23. They universally expressed satisfaction with the postoperative cosmetic outcomes.
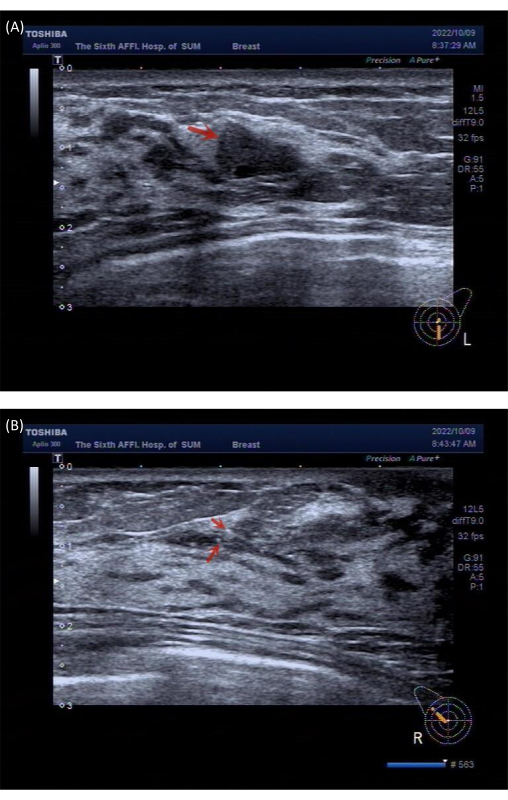
Figure 1: Preoperative ultrasound imaging data from the patient. (A) The image reveals a solid nodule located at the 6 o'clock position of the left breast. (B) The image reveals multiple calcifications located at the 10 o'clock position of the right breast. Please click here to view a larger version of this figure.
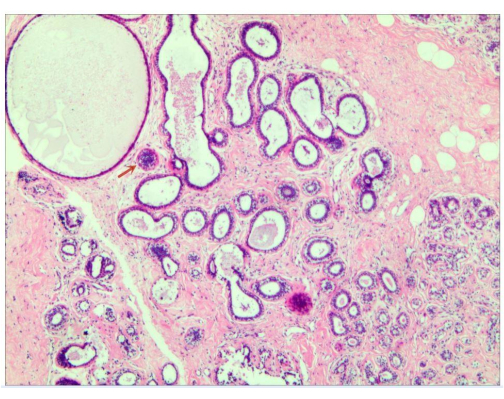
Figure 2: Preoperative histopathological result: The image shows ductal carcinoma in situ. Please click here to view a larger version of this figure.
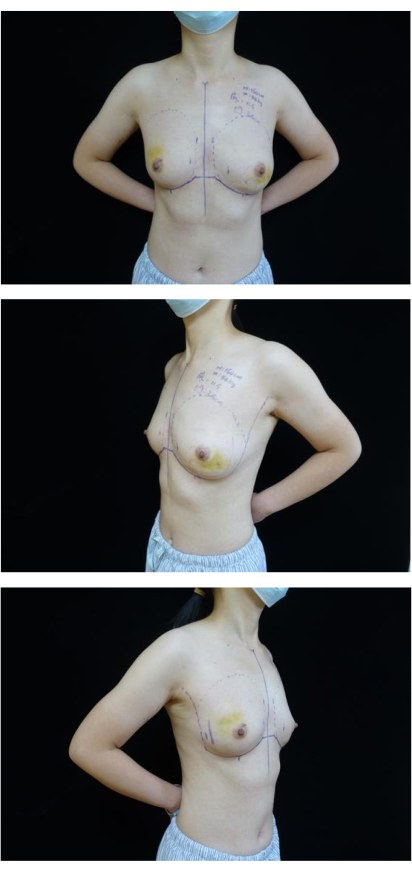
Figure 3: Preoperative marking. The images show the inferior fold of axilla, edge of breast and inframammary fold of the bilateral breasts were marked. Please click here to view a larger version of this figure.
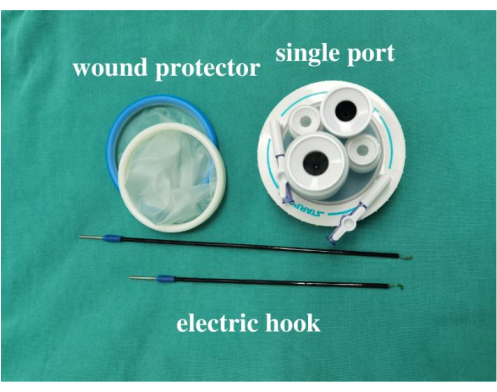
Figure 4: Surgical instruments. The image shows the wound protector, four-trocar single port, and electric hook. Please click here to view a larger version of this figure.
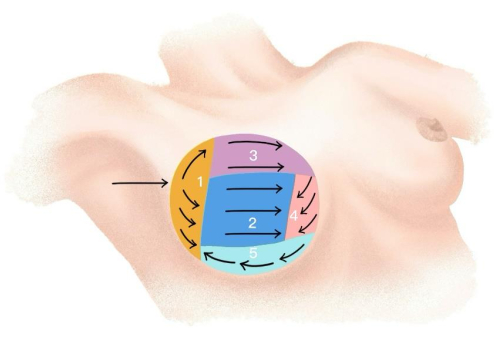
Figure 5: Dissection sequence. The image shows the dissection sequence in the retromammary space. Please click here to view a larger version of this figure.
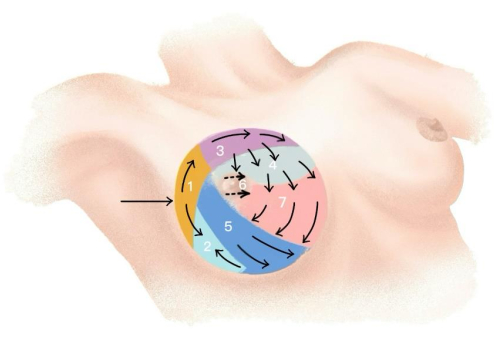
Figure 6: Zoning dissection sequence. The image shows the dissection sequence in the subcutaneous layer. Please click here to view a larger version of this figure.
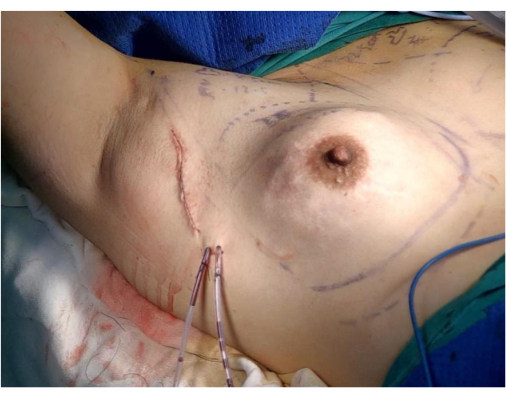
Figure 7: Postoperative result. The image shows that the implantation, drainage, and suture have been completed. Please click here to view a larger version of this figure.
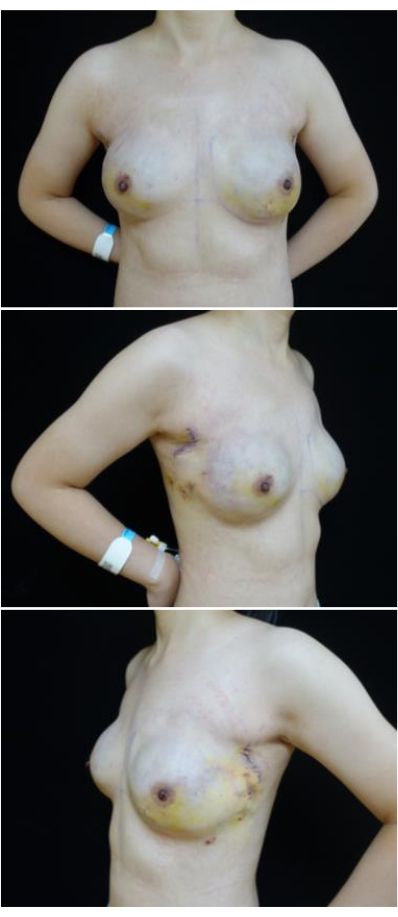
Figure 8: Postoperative photos. The images show the postoperative recovery of the patient after the removal of the drainage tubes on the 8th postoperative day. Please click here to view a larger version of this figure.
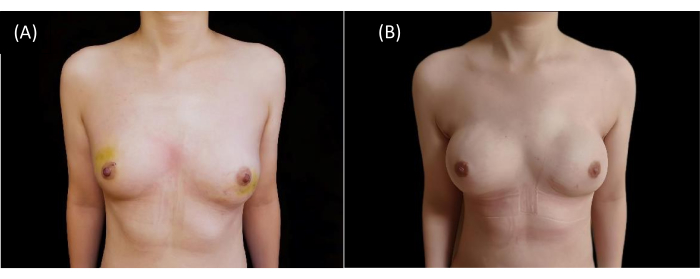
Figure 9: Postoperative cosmetic outcomes. (A) The image shows preoperative breast appearance. (B) The image shows postoperative cosmetic outcomes after 5 months. Please click here to view a larger version of this figure.
| Case | Age (years) | BMI (Kg/m2) | Operative time (h) | Blood loss (mL) | Postoperative hospital stay (days) | Operation reason |
| 1 | 50 | 20.20 | 4 | 20 | 16 | CPM |
| 2 | 42 | 25.97 | 3.5 | 20 | 11 | BBC |
| 3 | 49 | 27.34 | 4 | 20 | 19 | CPM |
| 4 | 34 | 19.56 | 4 | 50 | 7 | CPM |
| 5 | 45 | 20.31 | 4.5 | 50 | 15 | BBC |
| 6 | 51 | 22.03 | 4 | 50 | 6 | CPM |
| 7 | 33 | 18.75 | 4 | 20 | 10 | CPM |
| 8 | 45 | 29.38 | 6 | 20 | 10 | CPM |
| 9 | 36 | 16.53 | 4.5 | 50 | 10 | CPM |
| 10 | 33 | 21.09 | 4 | 30 | 10 | BBC |
| Average | 42 | 22.12 | 4.25 | 33 | 11 |
Table 1: Clinical parameters of 10 cases. Abbreviations: BMI=body mass index; CPM=contralateral prophylactic mastectomy; BBC=bilateral breast cancer.
Discussion
Represented by mastectomy, conventional breast surgery often yields unsatisfactory cosmetic outcomes and can adversely affect the functioning of the affected upper limb. This situation can cause considerable psychological distress, ultimately resulting in a reduced postoperative quality of life for patients24,25,26. Minimally invasive surgery represents a pivotal advancement in modern surgical practices. With the introduction of endoscopic techniques, significant improvements have been made to address the shortcomings of conventional surgery. Simultaneously, breast reconstruction has gained popularity as it offers the ability to restore the breast contour in breast cancer patients24. It has been shown to reduce the incidence of postoperative depression among breast cancer patients and enhance their postoperative quality of life27. This aligns with the emerging paradigm of the "biopsychosocial" medical model. With the integration of endoscopic techniques and breast reconstruction, breast surgery has entered a novel phase characterized by a focus on humanization and the optimization of patient outcomes. These objectives stand as the central tenets we strive for and uphold.
When compared with conventional E-NSM with IBBR, the benefits of endoscopic nipple-sparing mastectomy via a single axillary incision with immediate pre-pectoral implant-based breast reconstruction are evident. Firstly, this approach allows for the completion of all surgical procedures through a discreet single axillary incision, ensuring minimal visibility and achieving favorable postoperative cosmetic outcomes11,28,29. Moreover, the technique of E-NSM disrupts the vertical blood supply system to the nipple-areola complex (NAC), relying exclusively on the dermal vascular network. Avoiding incisions on the breast skin reduces the likelihood of NAC and flap ischemic necrosis8,30,31,32. In comparison to subpectoral reconstruction, pre-pectoral reconstruction is simpler, resulting in a more natural breast contour and minimizing chest functional deficits and pain33. However, in the context of pre-pectoral reconstruction, the absence of protection from the pectoralis major muscle means that direct contact between implants or meshes and the breast surface incision could lead to prosthetic exposure or even loss. Such complications may arise due to flap infection or ischemic necrosis34,35. Hence, the technique we present not only ensures the safety of tumor management but also yields superior cosmetic outcomes and a higher postoperative quality of life.
Several critical technical aspects warrant attention. First, it is imperative to preoperatively demarcate the extent of the patient's breast. Experienced surgeons can preoperatively manually mark the extent of the breast, while less experienced surgeons can employ preoperative ultrasound to delineate the breast boundaries. The circummammary ligaments are considered the natural boundaries of the breast and can serve as anatomical landmarks for breast dissection15,36. They can be observed both preoperatively with ultrasound and intraoperatively under endoscopic vision. Second, follow the dissection sequence from the retromammary space to the subcutaneous layer of the breast, which is recognized as a convenient and efficient strategy37. Third, while conducting the dissection of the retromammary space, meticulous attention is required when separating along the edges of the breast. This can be assessed through the utilization of dyes, finger press, and the pressure feedback sensation from endoscopic instruments. Whether through preoperative marking or employing techniques such as dyeing to delineate the breast boundaries, the central aim remains to guide surgeons to expose and identify the circummammary ligaments and precisely excise mammary glands. This is crucial for breast reconstruction surgery and contributes to achieving favorable postoperative cosmetic outcomes36. Fourth, it is advisable to ensure consistent flap thickness during the dissection of the subcutaneous layer within the breast. A technique we employ involves turning off the operating room lights and observing the extent to which the endoscope's light source penetrates through the breast's surface skin within the cavity, thereby assessing the uniformity of flap thickness.
There are some limitations to this method. First, even though E-NSM with IBBR has garnered extensive adoption and is considered a relatively mature technique, there remains a dearth of high-level evidence from evidence-based medicine to definitively establish its safety. Additionally, within our institution, we exclusively employ the single-port method for conducting endoscopic breast surgery. This decision is rooted in the fact that the lead surgeon has successfully overcome the learning curve and holds the belief that the single-port approach can reduce the number of incisions, obscure scars, and diminish the occurrence of ischemic necrosis in both the NAC and flap. Nevertheless, it's important to note that this perspective is subjective and currently lacks controlled studies to substantiate our standpoint.
In conclusion, when considering specific patients, the endeavor to enhance practice for improved cosmetic outcomes and heightened patient satisfaction holds significant value. This endeavor encapsulates the ethos of humanistic care and aesthetics. Our collective aspirations encompass the pursuit of beauty and the assurance of maximizing patient benefits.
Disclosures
No conflict of interest.
Acknowledgements
This work was supported by National Key Clinical Discipline.
Materials
| Name | Company | Catalog Number | Comments |
| Carbon nanoparticles suspension injection | LUMMY | H20041829 | |
| Drainage tube | Yangtze River | 20142140359 | |
| Electric hook | TNKJ | 20222010212 | |
| Endoscopic scissors | YOUSHI | P20200702015 | |
| 5-mm 35cm 30° rigid endoscope | Karl Storz | 26046BA | |
| Implant | MENTOR | 9718984-037 | |
| Laparoscopic grasping forceps | YOUSHI | P20200108013 | |
| Mesh | pfm medical titanium gmbh | 20163131539 | |
| Methylene blue | JUMPCAN | H32024824 | |
| 1 mL syringe (0.45×16RWLB) | WEGO | 20163141593 | |
| Single port | SURGAID | 5012151092 | |
| 10 mL syringe (0.8×38TWLB) | WEGO | 20163141593 |
References
- De La Cruz, L., Moody, A. M., Tappy, E. E., Blankenship, S. A., Hecht, E. M. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: A meta-analysis and systematic review. Ann Surg Oncol. 22 (10), 3241-3249 (2015).
- Howard, M. A., et al. Patient satisfaction with nipple-sparing mastectomy: A prospective study of patient reported outcomes using the breast-q. J Surg Oncol. 114 (4), 416-422 (2016).
- Orzalesi, L., et al. Nipple sparing mastectomy: Surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast. 25, 75-81 (2016).
- Fan, L. J., et al. A prospective study comparing endoscopic subcutaneous mastectomy plus immediate reconstruction with implants and breast conserving surgery for breast cancer. Chin Med J (Engl). 122 (24), 2945-2950 (2009).
- Mok, C. W., Lai, H. W. Endoscopic-assisted surgery in the management of breast cancer: 20 years review of trend, techniques and outcomes. Breast. 46, 144-156 (2019).
- Wan, A., et al. Association of long-term oncologic prognosis with minimal access breast surgery vs conventional breast surgery. JAMA Surg. 157 (12), e224711 (2022).
- Sakamoto, N., et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg Oncol. 16 (12), 3406-3413 (2009).
- Wuringer, E., Mader, N., Posch, E., Holle, J. Nerve and vessel supplying ligamentous suspension of the mammary gland. Plast Reconstr Surg. 101 (6), 1486-1493 (1998).
- Lai, H. W., et al. Current trends in and indications for endoscopy-assisted breast surgery for breast cancer: Results from a six-year study conducted by the taiwan endoscopic breast surgery cooperative group. PLoS One. 11 (3), e0150310 (2016).
- Lai, H. W., et al. Minimal access (endoscopic and robotic) breast surgery in the surgical treatment of early breast cancer-trend and clinical outcome from a single-surgeon experience over 10 years. Front Oncol. 11, 739144 (2021).
- Wang, X., et al. Trans-axillary single port insufflation technique-assisted endoscopic surgery for breast diseases: Clinic experience, cosmetic outcome and oncologic result. Front Oncol. 13, 1157545 (2023).
- Lai, H. W., et al. Single-axillary-incision endoscopic-assisted hybrid technique for nipple-sparing mastectomy: Technique, preliminary results, and patient-reported cosmetic outcome from preliminary 50 procedures. Ann Surg Oncol. 25 (5), 1340-1349 (2018).
- Xie, F., et al. Comparing outcomes of single-port insufflation endoscopic breast-conserving surgery and conventional open approach for breast cancer. World J Surg Oncol. 20 (1), 335 (2022).
- Wang, H., Wang, Q., Zhang, Y., Peng, Y. Value of ultrasound BIRADS classification in preoperative evaluation of the ultrasoundguided Mammotomeassisted minimally invasive resection of breast masses: A retrospective analysis. Exp Ther Med. 25 (4), 143 (2023).
- Rehnke, R. D., Groening, R. M., Van Buskirk, E. R., Clarke, J. M. Anatomy of the superficial fascia system of the breast: A comprehensive theory of breast fascial anatomy. Plastic & Reconstructive Surgery. 142 (5), 1135-1144 (2018).
- Suijker, J., Blok, Y. L., De Vries, R., Van Den Tol, M. P., Krekel, N. M. A. Pectoral fascia preservation in oncological mastectomy to reduce complications and improve reconstructions: A systematic review. Plast Reconstr Surg Glob Open. 8 (3), e2700 (2020).
- Chen, A. X., et al. Preservation of the pectoralis major fascia has no impact on the long-term oncologic outcomes of patients with breast cancer treated with conservative mastectomy and immediate breast reconstruction: A propensity score matching analysis. J Plast Reconstr Aesthet Surg. 86, 231-238 (2023).
- Duncan, A. M., et al. Anatomy of the breast fascial system: A systematic review of the literature. Plast Reconstr Surg. 149 (1), 28-40 (2022).
- De Almeida Rizzi, S. K. L., et al. Early free range-of-motion upper limb exercises after mastectomy and immediate implant-based reconstruction are safe and beneficial: A randomized trial. Ann Surg Oncol. 27 (12), 4750-4759 (2020).
- Field, D. A., Miller, S. Cosmetic breast surgery. Am Fam Physician. 45 (2), 711-719 (1992).
- Riddle, L. B. Expansion exercises: Modifying contracture of the augmented breast. Res Nurs Health. 9 (4), 341-345 (1986).
- Barker, D. E., Schultz, S. L. The theory of natural capsular contraction around breast implants and how to prevent it. Aesthetic Plast Surg. 4 (1), 357-361 (1980).
- Harris, J. R., Levene, M. B., Svensson, G., Hellman, S. Analysis of cosmetic results following primary radiation therapy for stages i and ii carcinoma of the breast. Int J Radiat Oncol Biol Phys. 5 (2), 257-261 (1979).
- Kaufman, C. S. Increasing role of oncoplastic surgery for breast cancer. Curr Oncol Rep. 21 (12), 111 (2019).
- Zwakman, M., et al. Long-term quality of life and aesthetic outcomes after breast conserving surgery in patients with breast cancer. Eur J Surg Oncol. 48 (8), 1692-1698 (2022).
- Sung, H., et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71 (3), 209-249 (2021).
- Stevens, L. A., et al. The psychological impact of immediate breast reconstruction for women with early breast cancer. Plast Reconstr Surg. 73 (4), 619-628 (1984).
- Lai, H. W., et al. Single-port three-dimensional (3d) videoscope-assisted endoscopic nipple-sparing mastectomy in the management of breast cancer: Technique, clinical outcomes, medical cost, learning curve, and patient-reported aesthetic results from 80 preliminary procedures. Ann Surg Oncol. 28 (12), 7331-7344 (2021).
- Franceschini, G., et al. Nipple-sparing mastectomy combined with endoscopic immediate reconstruction via axillary incision for breast cancer: A preliminary experience of an innovative technique. The Breast Journal. 26 (2), 206-210 (2019).
- Visconti, G., et al. Transaxillary nipple-sparing mastectomy and direct-to-implant breast reconstruction using a simplified endoscopic approach: Indications, cosmetic outcomes and technical refinements. Aesthetic Plastic Surgery. 44 (5), 1466-1475 (2020).
- Baker, B. G., et al. A prospective comparison of short-term outcomes of subpectoral and prepectoral strattice-based immediate breast reconstruction. Plast Reconstr Surg. 141 (5), 1077-1084 (2018).
- Sanson, C., et al. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: A prospective study of 138 procedures. Chirurgia (Bucur). 116 (2), 135-142 (2021).
- Sigalove, S., et al. Prepectoral implant-based breast reconstruction: Rationale, indications, and preliminary results. Plast Reconstr Surg. 139 (2), 287-294 (2017).
- Dave, R. V., et al. Risk factors for complications and implant loss after prepectoral implant-based immediate breast reconstruction: Medium-term outcomes in a prospective cohort. Br J Surg. 108 (5), 534-541 (2021).
- Highton, L., Johnson, R., Kirwan, C., Murphy, J. Prepectoral implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 5 (9), e1488 (2017).
- Matousek, S. A., Corlett, R. J., Ashton, M. W. Understanding the fascial supporting network of the breast: Key ligamentous structures in breast augmentation and a proposed system of nomenclature. Plast Reconstr Surg. 133 (2), 273-281 (2014).
- Zhang, S., et al. Video-assisted transaxillary nipple-sparing mastectomy and immediate implant-based breast reconstruction: A novel and promising method. Aesthetic Plast Surg. 46 (1), 91-98 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved