A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Experimental Model of Ligature-Induced Peri-Implantitis in Mice
In This Article
Summary
This is a report on an experimental model of ligature-induced peri-implantitis in mice. We describe all surgical steps, from pre- and post-operative management of the animals, extractions, implant placement, and ligature-induced peri-implantitis.
Abstract
Dental implants have a high success and survival rate. However, complications such as peri-implantitis (PI) are highly challenging to treat. PI is characterized by inflammation in the tissues around dental implants with progressive loss of supporting bone. To optimize dental implants' longevity in terms of health and functionality, it is crucial to understand the peri-implantitis pathophysiology. In this regard, using mouse models in research has proven clear benefits in recreating clinical circumstances. This study aimed to describe an experimental model of ligature-induced peri-implantitis in mice and determine whether there is effectiveness in inducing this disease, given the observed bone and tissue changes. The experimental peri-implantitis induction comprehends the following steps: teeth extraction, implant placement, and ligature-inducted PI. A sample of eighteen 3-week-old C57BL/6J male mice was divided into two groups, ligature (N=9) and control non-ligature (N=9). The evaluation of clinical, radiographical, and histological factors was performed. The ligature group showed significantly higher bone loss, increased soft tissue edema, and apical epithelial migration than the non-ligature group. It was concluded that this pre-clinical model can successfully induce peri-implantitis in mice.
Introduction
Dental implants are increasingly prevalent as a desirable choice for replacing missing teeth1. The prevalence of dental implants in the US adult population is projected to increase up to 23% by 20262. Based on a market analysis report by Grand View Research (2022), the global market size of dental implants was projected to reach approximately US $4.6 billion in 2022. Furthermore, it is anticipated to exhibit a steady annual growth rate of around 10% until the year 20303. Unfortunately, the use of dental implants can lead to complications, such as peri-implantitis. Peri-implantitis has been defined as a biofilm-induced condition characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone4.
A systematic review found that the mean prevalence of peri-implantitis was 19.53% (95% Confidence interval [CI], 12.87 to 26.19%) at the patient level and 12.53% (95% CI 11.67 to 13.39%) at the implant-level5. Peri-implantitis represents a growing public health, due to an increase in implant failure and, consequently, substantial treatment costs6.
Understanding the pathogenesis of peri-implantitis is crucial to developing a systematic approach to prevent its onset and progression and maximize dental implants' longevity in terms of aesthetics and function7,8. In this sense, using murine models in dental research has proven advantageous, given that mice share more than 95% of their genes with humans9,10, the number of available online genetic databases, and the ability to reproduce clinical scenarios11. All the described advantages allow the dissection of genetic mechanisms in different diseases12, accessible accommodation and management, and antibodies widely available as human panels, beyond the genetic modification availability (e.g., knockout and overexpression) for inflammatory tissue assessment and disease mapping13. Although advantageous, there are few publications addressing peri-implantitis in mice. This is due to methodological challenges, among others, including the difficulty in obtaining mini-implants or installing them.
To develop peri-implantitis in mice, many protocols have been described, such as ligature-induced peri-implantitis, bacteria-induced peri-implantitis14, Lipopolysaccharide (LPS)-induced peri-implantitis15, or the combination LPS + ligature-induced peri-implantitis16. Here, we will focus on the ligature model because it is the most widely accepted method to induce periodontitis17,18,19 and, more recently, peri-implantitis20,21. The ligature placed around the implants in a submucosal position stimulates plaque accumulation and, consequently, tissue inflamation. So, the development of this approach is based on the indication of a viable cost-benefit technique for pre-clinical investigations on peri-implant diseases. This study aims to describe an experimental model of ligature-induced peri-implantitis in mice and determine whether there is effectiveness in inducing this disease given the observed bone and tissue changes.
The overall goal of this article is to report the protocol applied to induce peri-implantitis in mice by ligature and to observe its effectiveness through tissue evaluation and bone loss around the implants.
Access restricted. Please log in or start a trial to view this content.
Protocol
Procedures involving animal subjects have been approved by the Chancellor's Animal Research Committee of the University of California, Los Angeles (ARC protocol number 2002-125), and the Animal Research: Reporting In Vivo Experiments (ARRIVE)22. For this method, eighteen 3-week-old C57BL/6J male mice were used and underwent dental extractions, implant placement and peri-implantitis induction. All dental procedures were performed under 10× microscopic magnification and carried out by trained and calibrated operators (Figure 1A).
1. Pre-extraction steps
- Perform the procedure in a surgical room complying with all biosafety and protection standards.
- Perform asepsis of all surfaces with triple friction using a 70% isopropyl alcohol solution.
- Anesthetize 3-week-old C57BL/6J male mice with 3% isoflurane. Check for the lack of response to paw withdrawing upon touch to ensure sufficient anesthetic depth is obtained.
- To avoid interfering with procedures in the mouth, use a nosecone to maintain isoflurane anesthesia.
- Have an auxiliary operator to stabilize the animal and maintain the mouth opening (Figure 1B).
- Apply ophthalmic lubricant, to prevent irritation in the eyes before starting the extraction.
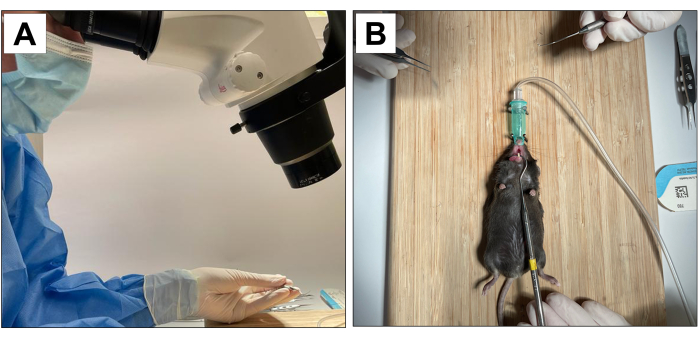
Figure 1: Operative adaptations: (A) Microscopic magnification. (B) Adapted inhalation anesthesia system and stabilization for mouth opening. Please click here to view a larger version of this figure.
2. Dental extraction
- For dental extractions, introduce a #5 dental explorer between the first and the second molar to start the elevation and luxation procedures by moving the instrument in the mesio-distal direction until the tooth moves in the socket (Figures 2A).
- Next, introduce the #5 dental explorer in the mesial site of the first molar. Do this by moving the instrument in the mesio-distal direction until the tooth moves in the socket.
- Following elevation, use the tip forceps and the suture tying forceps to remove the 1st molar.
- Next, introduce the dental explorer between the 2nd and 3rd molar to elevate and luxate the 2nd molar (Figure 2B).
- Then, use the tip forceps and/or the suture tying forceps to remove the tooth using the instruments to hold it and take out of the mouth. (Figure 2C).
- After dental extractions, ensure complete hemostasis achievement by using a sterile cotton tipped (Figure 2D) for 1 min (Figure 2E,F).
- Immediately after extraction, provide all animals with pain medication (Carprofen/Rimadyl 5 mg/kg) every 24 h. Administer the medication through subcutaneous injection.
- In addition, replace regular food with a soft diet. Administer the antibiotic (Amoxil 0.25 mg/mL) orally by incorporating the medication into the drinking water. Do this for four weeks after extractions.
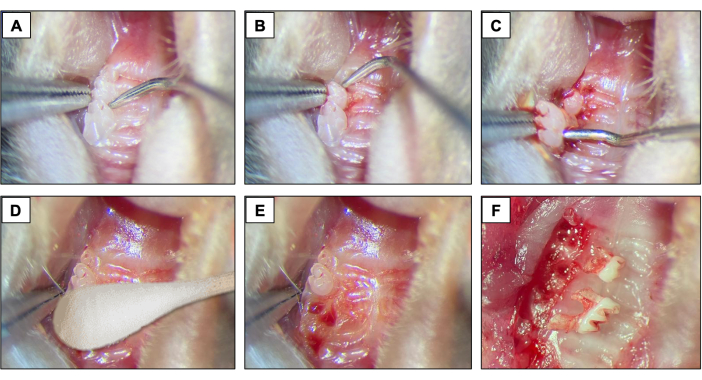
Figure 2: Initial extraction sequence: (A, B) Maxillary region with 1st and 2nd molar teeth and use of dental explorer for elevation and luxation. (C) Use of the tip forceps and explorer for luxation and tooth removal. (D) Hemostasis. (E, F) Alveolar appearance after extractions. Please click here to view a larger version of this figure.
3. Implant placement
- Using a 15c blade, create a mesio-distal incision through the keratinized tissue in the area corresponding to the previously present teeth. Use the right maxillary molars as a spatial reference (Figure 3A).
- Raise the buccal and palatal full-thickness flaps by using a #5 dental explorer, ensuring complete flap elevation (Figure 3B).
- Perform the osteotomy using a 0.3 mm diameter carbide micro hand drill attached to a Pin Vise (hand drill) and activate it in a clockwise rotation. Create osteotomy sites with approximately 1 mm deep into the healed extraction sockets (Figure 3C).
- Place the custom-designed screw-shaped implants with a smooth machined surface (1.0 mm long and 0.5 mm in diameter) fabricated from 6AL4V titanium rods (Figure 3D), one per animal, by self-tapping in the region of the first and second maxillary left molars using a clockwise screwing motion (Figure 3E-I).
- Immediately after implant placement, administer pain medication (Carprofen/Rimadyl 5mg/kg every 24 h) through subcutaneous injection.
- Allow the implants to heal for four weeks, during which time administer antibiotics and feed as previously described.
- At the end of the healing period, ensure that the mucosal wound is completely closed and has a light pink appearance.
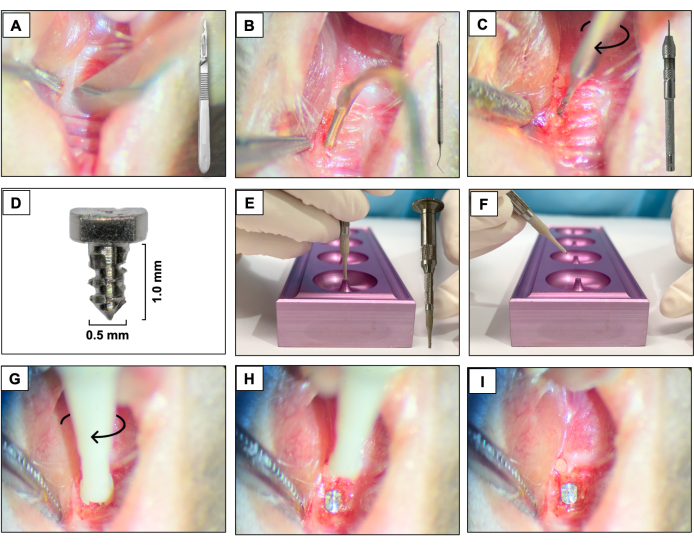
Figure 3: Implant placement sequence: (A) Incision using a 15c blade attached to the handle. (B) Full-thickness flaps using #5 dental explorer. (C) Osteotomy using a 0.3 mm carbide micro hand drill attached to a Pin Vise. (D) Titanium dental implant. (E, F) Implant support and implant holder. (G- I) Implant placement using a clockwise screwing motion. Please click here to view a larger version of this figure.
4. Peri-implantitis induction
- Place a silk ligature (6-0) around each fixture immediately apical to the implant head by contouring the surface of the implant and fixing it with a double knot. Keep the ligature for two weeks allowing the peri-implantitis development.
- Check the ligatures every two days to make sure they are still present. If missing, place a new ligature.
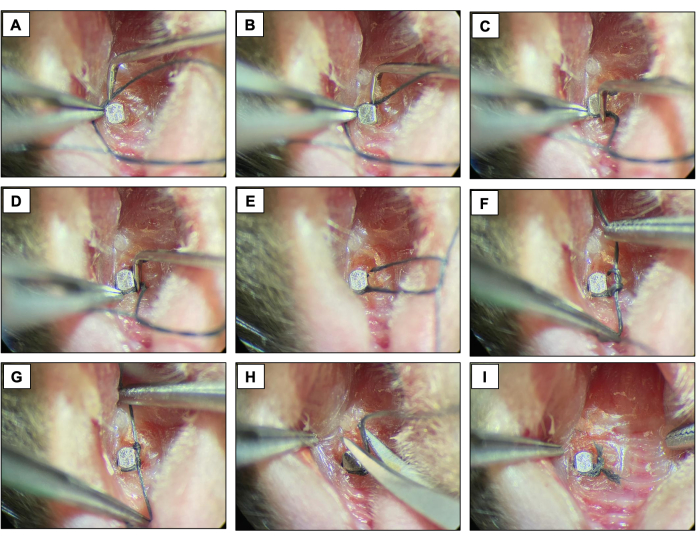
Figure 4: Ligature-induced peri-implantitis sequence. (A-D) Silk ligature (6.0) placed around the implant head. (E-G) Knot closure. (H) Ligature cut. (I) Final appearance. Clinical images obtained from live animals under sedation. Please click here to view a larger version of this figure.
5. Sacrifice
- After the peri-implantitis induction period (two weeks), sacrifice all animals with a high concentration of inhaled isoflurane, followed by a secondary method of cervical dislocation with a rod pressed into the base of the skull and quickly pulling the hind limbs, causing the separation of the cervical vertebrae from the skull.
- Separate the maxillae using sharp instruments, such as surgical scissors, that will separate the animal's entire jaw from the rest of the body.
- Photograph the maxillae using an optical microscope, fix it in 10% formalin for 24 h, then store in 70% ethanol.
6. Micro-computed tomography (µCT )
- Scan the maxillae as previously described18,19 using micro-computed tomography (micro-CT) scanning.
- Turn on the scanner and the computer, then open the scan software, click on the radiation sign, and wait 15 minutes to preheat it.
- Open the equipment door by clicking on the door icon.
- Use a quarter-size rotary table. Place the maxilla on a 15ml conical tube. Fix the tube in the rotary table base. Place the rotary table in the equipment screw.
- Close the equipment door by clicking on the door icon.
- Turn on the X-Ray source.
- Click on the grab image icon and make sure the bone is on the field.
- Adjust a resolution of 10 µm pixel size, X-ray energy of 55 KVp and 181 µA, filter of AI 0.5 mm, rotation step 0.4, average frame 10. These settings will generate an estimated scanning time of 24 minutes.
- Start the acquisition.
- For reconstruction open the reconstruction software. Use beam hardening of 20%. Use ring artifact of 5%. Use a dynamic range of 0-0.13. Deselect label ON. Click on Preview. Choose the adequate view that is seen for the region of interest, and save it as TIFF.
- Orient the images obtained using software for viewing and analyzing data. Make the long axis of the implant head parallel to the sagittal and coronal axes and perpendicular to the axial axis.
- Save the sagittal image as a single image for linear bone analysis.
- Save transaxial image as a dataset for volumetric bone analysis.
- Perform linear bone analysis using the analyzing data software. Measure the distances in millimeters between the implant head and the alveolar bone in the sagittal and coronal planes, including mesial, distal, buccal, and palatal sites.
- Perform volumetric bone analysis using analyzing data software. Track bone loss around implants by drawing the region of interest in all slides corresponding to the bone loss.
7. Statistical analysis
- Measure linear and volumetric microtomographic bone loss and normalize these values by dividing each value by the average of the control group. Present an average for all groups, as mean ± standard error of the mean (SEM).
- Compare significance using a two-way analysis of variance (ANOVA) followed by a Tukey's test with a 95% confidence interval. (Prism 5). Apply significance levels as follows: p≤0.01**, p≤0.001****.
Access restricted. Please log in or start a trial to view this content.
Results
For this method, eighteen 3-week-old C57BL/6J male mice were used and underwent dental extractions, implant placement and peri-implantitis induction. There were nine animals per group which was statistically significant, considering linear bone loss achieving 80% power, 15% standard deviation (σ) and 95% confidence interval (α =0.05). Mice were fed a soft diet ad libitum during the experiment. Nine mice received a ligature (ligature-induced periimplantitis-experimental group), and nine mice did not rec...
Access restricted. Please log in or start a trial to view this content.
Discussion
This protocol presents a descriptive report on surgical procedures for peri-implantitis induction utilizing a ligature model in mice. Working with mice has advantages, such as being cost-effective, the availability of an extensive genetic array given the many backgrounds23 among other aspects24,25. Over the years, several studies have successfully utilized mice in the medical and dental fields, including in peri-implantitis
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the NIH/NIDCR DE031431. We would like to thank the Translational Pathology Core Laboratory at UCLA for assistance with preparing the decalcified histological sections.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| #5 dental explorer | Hu-Friedy, Chicago, IL | 392-0911 | Dental luxation |
| 15c blade and surgical scalpel | Henry Schein Inc., Melville, NY | 1126186 | Tissue incision |
| 6-0 silk ligatures | Fisher Scientific, Hampton, NH | NC9201232 | Ligature |
| Amoxicillin 50μg/mL | Zoetis, San Diego, CA | TS/DRUGS/57/2003 | Oral suspension |
| Bacon Soft Diet | Bio Serve®, Frenchtown, NJ | 14-726-701 | - |
| C57BL/6J male mice | The Jackson Laboratories, Bar Harbor, ME, USA | 000664 | Age: 3-week-old |
| CTAn software | V.1.16 Bruker, Billerica, MA | - | Volumetric analysis |
| Dolphin software | Navantis, Toronto, CA | - | Linear bone analysis |
| Implant carrier & Tip | D. P. Machining Inc., La Verne, CA | Unique product | Implant holder |
| Implant support | D. P. Machining Inc., La Verne, CA | Unique product | Implant capture |
| Isoflurane | Vet One, Boise, ID | NDC13985-528-60 | Inhalational anesthetic |
| Micro-CT scan 1172 | SkyScan, Kontich, Belgium | - | μCT scans |
| Nrecon Software | Bruker Corporation, Billerica, MA | - | Images reconstruction |
| Ø 0.3mm - L 2.5mm Micro Drills | Sphinx, Hoffman Estates, IL | ART. 50699 | Osteotomy |
| Ø 0.5mm - L 1.0mm Titanium implants | D. P. Machining Inc., La Verne, CA | Unique product | - |
| Ophthalmic lubricant | Apexa, Ontario, CA | NDC13985-600-03 | Artificial tears |
| Pin Vise | General Tools, Secaucus, NJ | 90 | Osteotomy |
| Rimadyl 50mg/ml | Zoetis, San Diego, CA | 4019449 | Anti-inflammatory |
| Sterile cotton tipped | Dynarex, Glendale, AZ | 4304-1 | Hemostasis |
| Tip forceps | Fine Science Tools, Foster City, CA | 11071-10 | Dental Extraction |
| Tying forceps | Fine Science Tools, Foster City, CA | 18025-10 | Ligature placement |
References
- Ho, K., et al. A cross-sectional survey of patient's perception and knowledge of dental implants in japan. Int J Implant Dent. 8 (1), 14(2022).
- Elani, H. W., Starr, J. R., Da Silva, J. D., Gallucci, G. O. Trends in dental implant use in the u.S., 1999-2016, and projections to 2026. J Dent Res. 97 (13), 1424-1430 (2018).
- Grand view research. , Available from: grandviewresearch.com/industry-analysis/dental-implants-market (2022).
- Renvert, S., Persson, G. R., Pirih, F. Q., Camargo, P. M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J Clin Periodontol. 45, Suppl 20 S278-S285 (2018).
- Diaz, P., Gonzalo, E., Villagra, L. J. G., Miegimolle, B., Suarez, M. J. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health. 22 (1), 449(2022).
- Herrera, D., et al. Prevention and treatment of peri-implant diseases-the efp s3 level clinical practice guideline. J Clin Periodontol. 50, Suppl 26 4-76 (2023).
- Graziani, F., Figuero, E., Herrera, D. Systematic review of quality of reporting, outcome measurements and methods to study efficacy of preventive and therapeutic approaches to peri-implant diseases. J Clin Periodontol. 39, Suppl 12 224-244 (2012).
- Schwarz, F., Derks, J., Monje, A., Wang, H. L. Peri-implantitis. J Periodontol. 89, Suppl 1 S267-S290 (2018).
- Bryda, E. C. The mighty mouse: The impact of rodents on advances in biomedical research. Mo Med. 110 (3), 207-211 (2013).
- Mouse Genome Sequencing, C. Et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 420 (6915), 520-562 (2002).
- Pirih, F. Q., et al. Ligature-induced peri-implantitis in mice. J Periodontal Res. 50 (4), 519-524 (2015).
- Rau, C. D., et al. High-density genotypes of inbred mouse strains: Improved power and precision of association mapping. G3. 5 (10), Bethesda. 2021-2026 (2015).
- Schwarz, F., Sculean, A., Engebretson, S. P., Becker, J., Sager, M. Animal models for peri-implant mucositis and peri-implantitis. Periodontol 2000. 68 (1), 168-181 (2015).
- Varon-Shahar, E., et al. Peri-implant alveolar bone resorption in an innovative peri-implantitis murine model: Effect of implant surface and onset of infection. Clin Implant Dent Relat Res. 21 (4), 723-733 (2019).
- Pirih, F. Q., et al. A murine model of lipopolysaccharide-induced peri-implant mucositis and peri-implantitis. J Oral Implantol. 41 (5), e158-e164 (2015).
- Schwarz, F., et al. Influence of antiresorptive/antiangiogenic therapy on the extension of experimentally induced peri-implantitis lesions. Clin Oral Investig. 27 (6), 3009-3019 (2023).
- Wong, R. L., et al. Comparing the healing potential of late-stage periodontitis and peri-implantitis. J Oral Implantol. 43 (6), 437-445 (2017).
- Wong, R. L., et al. Early intervention of peri-implantitis and periodontitis using a mouse model. J Periodontol. 89 (6), 669-679 (2018).
- Hiyari, S., et al. Ligature-induced peri-implantitis and periodontitis in mice. J Clin Periodontol. 45 (1), 89-99 (2018).
- Nguyen Vo, T. N., et al. Ligature induced peri-implantitis: Tissue destruction and inflammatory progression in a murine model. Clin Oral Implants Res. 28 (2), 129-136 (2017).
- Yuan, S., et al. Comparative transcriptome analysis of gingival immune-mediated inflammation in peri-implantitis and periodontitis within the same host environment. J Inflamm Res. 15, 3119-3133 (2022).
- Berglundh, T., et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 89, Suppl 1 S313-S318 (2018).
- Hiyari, S., et al. Genomewide association study identifies cxcl family members as partial mediators of lps-induced periodontitis. J Bone Miner Res. 33 (8), 1450-1463 (2018).
- Kantarci, A., Hasturk, H., Van Dyke, T. E. Animal models for periodontal regeneration and peri-implant responses. Periodontol 2000. 68 (1), 66-82 (2015).
- Struillou, X., Boutigny, H., Soueidan, A., Layrolle, P. Experimental animal models in periodontology: A review. Open Dent J. 4, 37-47 (2010).
- Erata, E., et al. Cnksr2 loss in mice leads to increased neural activity and behavioral phenotypes of epilepsy-aphasia syndrome. J Neurosci. 41 (46), 9633-9649 (2021).
- Fakih, D., Guerrero-Moreno, A., Baudouin, C., Reaux-Le Goazigo, A., Parsadaniantz, S. M. Capsazepine decreases corneal pain syndrome in severe dry eye disease. J Neuroinflammation. 18 (1), 111(2021).
- Douam, F., Ploss, A. The use of humanized mice for studies of viral pathogenesis and immunity. Curr Opin Virol. 29, 62-71 (2018).
- Lin, P., et al. Application of ligature-induced periodontitis in mice to explore the molecular mechanism of periodontal disease. Int J Mol Sci. 22 (16), (2021).
- Marchesan, J., et al. An experimental murine model to study periodontitis. Nat Protoc. 13 (10), 2247-2267 (2018).
- Silva, D. N. A., et al. Probiotic lactobacillus rhamnosus em1107 prevents hyperglycemia, alveolar bone loss, and inflammation in a rat model of diabetes and periodontitis. J Periodontol. 94 (3), 376-388 (2023).
- Kim, Y. G., et al. 6-shogaol, an active ingredient of ginger, inhibits osteoclastogenesis and alveolar bone resorption in ligature-induced periodontitis in mice. J Periodontol. 91 (6), 809-818 (2020).
- Fine, N., et al. Periodontal inflammation primes the systemic innate immune response. J Dent Res. 100 (3), 318-325 (2021).
- Yu, X., et al. Role of toll-like receptor 2 in inflammation and alveolar bone loss in experimental peri-implantitis versus periodontitis. J Periodontal Res. 53 (1), 98-106 (2018).
- Reinedahl, D., Chrcanovic, B., Albrektsson, T., Tengvall, P., Wennerberg, A. Ligature-induced experimental peri-implantitis-a systematic review. J Clin Med. 7 (12), (2018).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved