A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Behavioral Assay to Measure Responsiveness of Zebrafish to Changes in Light Intensities
In This Article
Summary
We developed the Visual-Motor Response to quantitate the motor output of larval zebrafish in response to light increments and decrements. We also examined zebrafish vision mutants, including the no optokinetic response (nrc) mutants, which were thought to be completely blind when tested by another vision assay, the optokinetic reflex.
Abstract
The optokinetic reflex (OKR) is a basic visual reflex exhibited by most vertebrates and plays an important role in stabilizing the eye relative to the visual scene. However, the OKR requires that an animal detect moving stripes and it is possible that fish that fail to exhibit an OKR may not be completely blind. One zebrafish mutant, the no optokinetic response c (nrc) has no OKR under any light conditions tested and was reported to be completely blind. Previously, we have shown that OFF-ganglion cell activity can be recorded in these mutants. To determine whether mutant fish with no OKR such as the nrc mutant can detect simple light increments and decrements we developed the visual motor behavioral assay (VMR). In this assay, single zebrafish larvae are placed in each well of a 96-well plate allowing the simultaneous monitoring of larvae using an automated video-tracking system. The locomotor responses of each larva to 30 minutes light ON and 30 minutes light OFF were recorded and quantified. WT fish have a brief spike of motor activity upon lights ON, known as the startle response, followed by return to lower-than baseline activity, called a freeze. WT fish also sharply increase their locomotor activity immediately following lights OFF and only gradually (over several minutes) return to baseline locomotor activity. The nrc mutants respond similarly to light OFF as WT fish, but exhibit a slight reduction in their average activity as compared to WT fish. Motor activity in response to light ON in nrc mutants is delayed and sluggish. There is a slow rise time of the nrc mutant response to light ON as compared to WT light ON response. The results indicate that nrc fish are not completely blind. Because teleosts can detect light through non-retinal tissues, we confirmed that the immediate behavioral responses to light-intensity changes require intact eyes by using the chokh (chk) mutants, which completely lack eyes from the earliest stages of development. In our VMR assay, the chk mutants exhibit no startle response to either light ON or OFF, showing that the lateral eyes mediate this behavior. The VMR assay described here complements the well-established OKR assay, which does not test the ability of zebrafish larvae to respond to changes in light intensities. Additionally, the automation of the VMR assay lends itself to high-throughput screening for defects in light-intensity driven visual responses.
Protocol
This protocol outlines the steps to perform the Visual-Motor Response of zebrafish larvae to light increments and decrements in your own lab. Zebrafish are a great model system for behavioral studies. They are easy to maintain, they have large clutch sizes, and they develop quickly. For example, the eyes of zebrafish larvae are responsive to light by day 3 of development and at which time they exhibit a startle response.
Part 1: Plating individual fish into a 96 well plate
- Grow WT larvae under a dark/light cycle at 28°C until at least 4 days post fertilization (dpf). Our typical light:dark cycle is 14 hours of lights ON starting at 9:00AM, and 10 hours of lights OFF, starting at 11:00 PM. For the best behavioral results, avoid overcrowding; we usually keep no more than 50 larvae in a single petri dish.
- After 4 dpf, the zebrafish are ready to be transferred into a 96-well plate. To give the larvae more swimming room, we typically use a 96-well plate with a large well-size of 650 µl; however, standard 96-well plates work just fine. Using a plastic transfer pipet, gently transfer one larva per well.
- After transferring fish into the wells, fill out each well with enough fish water such that the water surface is nearly flush to the top of the wells. Either overfilling or underfilling the well can cause optical problems for the recording camera. Also, take care not to introduce bubbles into the wells.
Part 2: Survey of the recording apparatus
For this part of the protocol, please refer to the film to identify components and to familiarize yourself with our set up of the recording apparatus.
- Inside the recording chamber is a well defined place to position the 96-well plate.
- The camera is positioned in the back and is focused on the plate using mirrors off the box. The angle of these mirrors can be adjusted by turning the screws that hold the mirrors in place.
- The recording chamber is illuminated from the bottom by infrared LEDs. This allows the camera to see the fish even in the dark. The larvae cannot detect IR light, so this constant IR illumination does not affect the experiment. White LEDs also illuminate the recording chamber from below. They are controlled separately from the IR lights. Illuminating the chamber with white light from above or from the sides is certainly possible; however, in those cases, care must be taken to avoid strong glares off the water surface that may interfere with the camera.
- For experiments that last longer than a couple of hours, fill the chamber with gently running water to help maintain a constant temperature for the experimental duration. One way to accomplish a constant flow of water is to pump water from a reservoir by a small aquarium pump that is heated to 28° with a typical underwater aquarium heater.
- To minimize stray vibration from the room, the entire recording unit should sit atop a heavy balance table.
Part 3: Alignment of the 96-well plate with the computer grid of the video-tracking
- Place the 96-well plate containing the fish into the recording chamber.
- When using a water bath, slowly place the plate in the water, giving the water level a chance to adjust without spilling onto the plate. Alternatively, shut off the water flow, add the plate, and then resume the flow. Also, be sure to use a spring or a rubber band to hold the 96-well plate in place.
- In the Viewpoint Videotrack software, check that all the larvae of your experiment are visible on the computer screen. Using the controls of the software, align the grid of the video-tracking software with the wells of your plate such that each fish is within a square of the grid. The tracking computer will be calculating the movement separately for each of these boxes, so if you misalign the computer grid some of the fish movements may be lost. Or even worse: two adjacent fish will occupy the same area and be counted as one fish. This step is very important for all your recordings.
- After the alignment, program the timing for when the lights should go on and off. We typically allow 3 hours of light or dark adaptation in the box both to obtain a baseline activity level, but also to give the larvae an opportunity to calm down following the pipetting and handling. After the baseline, we then alternate with 30 minutes of lights ON followed by 30 minutes of lights OFF, and repeat several times.
- Next, close the door of the recording chamber and start recording.
- In practice, we record the activity of each fish per second, but the Viewpoint software in fact records the frame by frame data (refer to the film for a demonstration of the data collection). Setting the threshold value for minimum pixel change per frame will depend somewhat on your particular camera and light setup. For our setup, we typically use a threshold of 4 pixels; that is, if fewer than 4 pixels are changing, it is considered background. If more than 4 pixels are moving, it indicates the fish is moving. We empirically determined that this cutoff detects nearly all the larval swim and turn movements.
- Even though the recording boxes isolate the larvae fairly well, turn OFF the light in the room and take care to minimize interruptions with mechanical noise such as closing and opening doors in the room, having a dance party, or doing your exercise routines.
Part 4: Analyzing the data
- After the experiment is completed, transfer the data collected into an excel sheet or into your favorite analysis suite.
- The attached excel sheet is an example of what the data may look like: it contains the time in seconds from the start of the experiment and the activity of each larva per seconds for all the fish throughout the entire experiment (See supplementary file Example Data in the Files section of this page).
- Figure 1 shows an example of the activity of a single larva. Figure 2 is a representative trace of the average of 40 WT zebrafish larvae. The averaged ON and OFF responses are prominent and consistent.
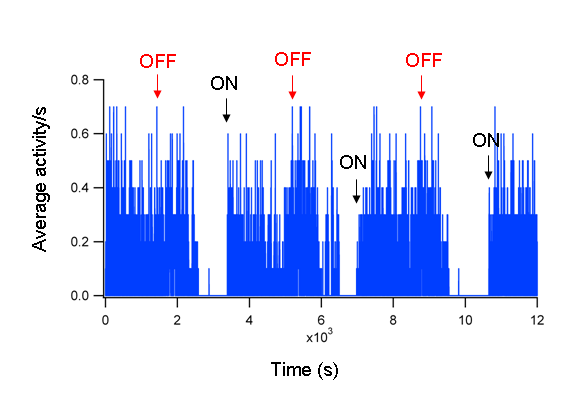
Figure 1: Activity of a single fish. The activity of a single WT fish at 5 dpf in response to alternating periods of 30 minutes light ON and OFF. The ON responses are indicated by black arrows and the OFF responses with red arrows.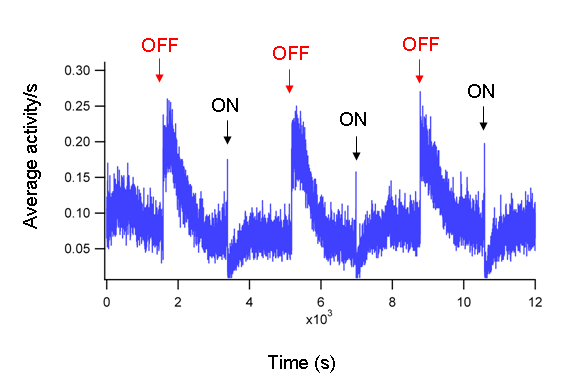
Figure 2: Average activity of 40 fish. The average activity of 40 WT fish at 5 dpf in response to alternating periods of 30 minutes light ON and OFF. The averaged ON (black arrows) and OFF (red arrows) responses are prominent and consistent.
5. Representative Results
Figure 3 is a schematic outline of the experimental outline used in all of our experiments.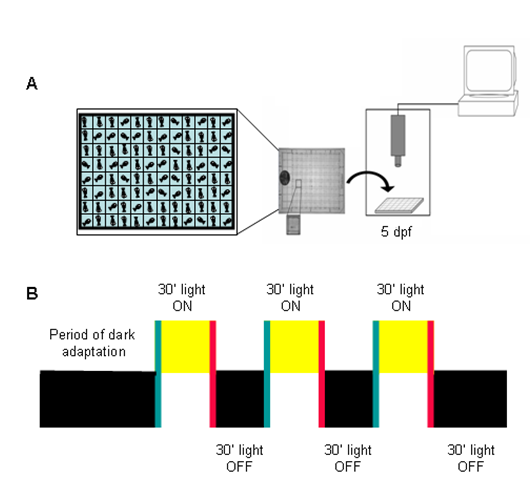
Figure 3. Experimental design of the Visual Motor Response (VMR) test. A) Individual fish are placed in a 96-well plate in a recording chamber. The activity of each fish is measured per second. B) Fish are given a period of dark or light adaptation to settle them and to obtain a baseline activity level. Periods of 30 minutes lights ON and lights OFF are introduced consecutively for a total of 3 hours. This diagram was adapted from Prober et al., 2006.
What do the Visual-Motor Response graphs look like?
We measured the ON and OFF responses of WT fish to light increments and decrements. To confirm that these responses were dependent on eye function, we measured the activity of chk mutants, which do not develop any eyes. Figure 4 shows the average activity obtained from WT animals as well as the chk mutants.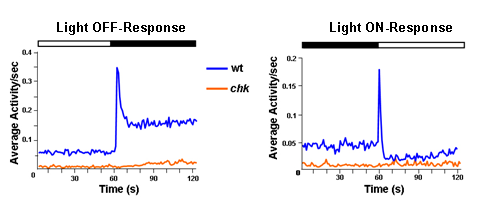
Figure 4: WT fish have clear ON and OFF responses that are mediated by the lateral eyes. The locomotor behavior of zebrafish larvae at 5 dpf in response to 30 minutes of light ON and 30 minutes of light OFF is recorded per second. Each trace represents an average of 480 responses from 120 individual WT (blue trace) or chk mutant larvae (orange trace) recorded over 3 experiments. The chk mutants do not significantly increase their activity to either light increments or decrements and have a low baseline level of activity. This figure was adapted from Emran et al., 2007.
Visual-Motor Responses from the nrc mutant fish.
The nrc mutant was thought to be completely blind based on the OKR test. In the nrc mutant, the photoreceptor terminals do not form properly and the On visual pathway is severely compromised 1. Retinal ganglion cell recordings from these mutant fish revealed that they exhibit predominantly OFF-type ganglion cell responses, some abnormal ON-OFF, but no pure On-type responses 2.
Using the VMR test we showed that the nrc mutant has a normal OFF-response and a delayed and sluggish ON-response (see Figure 5). Thus, the nrc mutant is not completely blind as previously thought 2.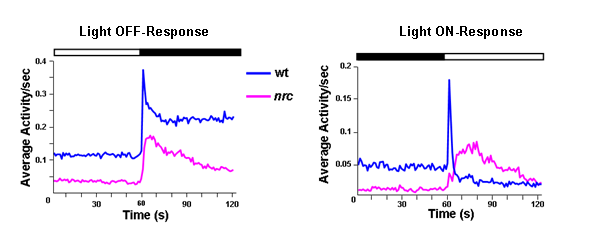
Figure 5: Nrc mutats increase their activity in response to changes in light intensities. Behavioral responses to light ON and OFF from WT and nrc mutant larvae at 5 dpf. Each trace represents an average of 480 responses from 120 individual fish from each genotype. The average locomotor behavior of nrc mutants (pink traces) is slightly reduced as compared to WT fish (blue traces) but remains vigorous following the light OFF stimulus. Note the slow rise time in the nrc mutant response to light ON compared to light ON response of the WT fish.
Discussion
The experimental procedures we show in the film are all representative of WT fish. However, these experiments can be done analogously on mutant fish as well (see representative result section). We suggest to plate WT fish and mutant fish on the same plate in a checker board outline for optimal control purposes.
When using mutant fish on the same plate as WT fish make sure that you write down what type of fish was plated in each well. Here is a suggestion on how to keep track of the fish plate...
Acknowledgements
This work was supported by National Institute of Health Grants EY0081 and 5T32UY07145 and by the Knights Templar Eye Foundation. Jason Rihel is a Bristol-Squibb Fellow of the Life Sciences Research Foundation.
Materials
| Name | Company | Catalog Number | Comments | |
| Microplate devices | Tool | Whatman, GE Healthcare | 7701-1651 | |
| Transfer pipetes | Tool | VWR international | 202205 | |
| Fish water | Reagent | refer to reference #4 | ||
| Recording chambers (Zebrabox) | Tool | Viewpoint Lifesciences | ||
| Videotrack Software | Tool | Viewpoint Lifesciences |
References
- Allwardt, A. B., Lall, B. A., Brockerhoff, S. E. Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J Neurosci. 21, 2330-2330 (2001).
- Emran, F., Rihel, J., Adolph, A. R. OFF ganglion cells cannot drive the optokinetic reflex in zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 104, 19126-19126 (2007).
- Prober, D. A., Rihel, J., Onah, A. A. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 26, 13400-13400 (2006).
- Westerfield, M. . The zebrafish book: a guide for the laboratory use of zebrafish. , (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved