Blood Withdrawal I
Overview
Source: Kay Stewart, RVT, RLATG, CMAR; Valerie A. Schroeder, RVT, RLATG. University of Notre Dame, IN
Blood collection is a common requirement for research studies that involve mice and rats. The method of blood withdrawal in mice and rats is dependent upon the volume of blood needed, the frequency of the sampling, the health status of the animal to be bled, and the skill level of the technician.1 All methods discussed-retro-orbital sinus bleeds, initial tail snip bleeds, and intracardiac bleeds-require the use of a general anesthesia.
Principles
Prior to the bleeding procedure, the type of sample required must be determined. Experimental procedures could require whole blood, plasma, or serum. For whole blood, an anticoagulant must be added to the sample. Plasma, which contains fibrinogen and other clotting factors when separated from the red blood cells, can be extracted from an anticoagulated sample. Serum is obtained through blood collection without an anticoagulant. The serum will result from centrifugation of the sample once a clot has formed. As the sample has clotted, the serum will not contain fibrinogen or other clotting factors. Both plasma and serum are obtained through the use of a centrifuge run at 2200-2500 RPM for a minimum of 15 minutes.
For a sample that must yield whole blood or plasma, an appropriate anticoagulant must be used. Commonly used anticoagulants for laboratory animals are heparin, sodium citrate, and ethylenediamine tetraacetic acid (EDTA); selection of which is based on research needs. Sequester-a liquid form of EDTA, heparin, and sodium citrate-can be loaded directly into the syringe to coat the surfaces. This allows contact of the anticoagulant directly as the blood is drawn, aiding in the prevention of clotting. As rat blood clots faster than most mammalian blood, it is essential that the correct ratio of anticoagulant to blood be used for blood collection.
Needle selection is based on the size of the animal and the site of the venipuncture. In general, the larger the bore of the needle, the more rapidly the sample can be collected. Less damage to the blood cells is another benefit to larger needles. However, the main disadvantage to large-bore needles is the potential damage to the vessel. On mice and rats, the choices of size range from 20-29 gauge needles that are 0.5-1.5 inches in length. If a needle is too long, not only is it awkward to use, but having the extra space in the needle could result in clotting. The appropriate needle size is listed for each method in the procedures section.
The size of the required sample must also be predetermined. Due to the small size of the mouse or rat, the maximum amount of blood collection must be calculated for a survival bleed. An average mouse weighing 25 grams has a total blood volume of 1.8 ml; the average rat weighing 250 grams has a total blood volume of 16 ml. For a single blood sample on a mouse or rat without fluid replacement, the maximum blood volume that can be safely removed is 10% of the total blood volume, or 7.7-8 µl/g. Thus for an average mouse, 10% of its blood volume is 193-200 µl. For an average rat of 250 grams, this is equivalent to 1.9-2.0 ml. Studies have shown that removing more than 15% of the blood volume can cause hypovolemic shock.1,2 However, with fluid replacement, up to 15% of the total blood volume-or 12 µl/g-can be removed. For a 25 gram mouse, this is equivalent to 300 µl; for a 250 gram rat, it is equivalent to 3 ml. For fluid replacement, the fluids should be warmed and given subcutaneously.
If it is necessary to take multiple samples, the blood volume drawn is reduced. The maximum blood volume that may be drawn per week is no more than 7.5% of the total blood volume, or 6 µl/g. For a 25 gram mouse, this is equivalent to 145-150 µl per week. For a 250 gram rat, this is equivalent to 1.45-1.50 ml per week. If sampling will occur every 2 weeks, up to 10% of the total blood volume (8 µl/g) may be drawn. This is equivalent to 200 µl every 2 weeks for an average mouse, and up to 2.00 ml every 2 weeks for a 250 gram rat. One study, performed on rats with the average weight of 250 grams, revealed that when blood volumes of 15-20% were removed, it took more than 29 days for blood levels to normalize.1,2 For repeated blood collection, fluid replacement does not allow for a larger blood volume or more frequent blood collection, as it only replaces volume. The animal will need time to replenish blood cells.
The use of the retro-orbital plexus has been a common practice in the past. However, many concerns about the humaneness of this procedure have arisen. During the procedure, excessive movement of the hematocrit tube once placed in the medial canthus of the eye can cause damage to the surrounding tissues, resulting in swelling of the eyelids and/or conjunctival membranes. The swollen tissues can cause the eyeball to protrude far enough so that closure of the eyelid is impeded, potentially resulting in corneal drying and damage. Pain from swelling can trigger scratching and self-mutilation that results in enucleation of the eye. Improper placement of the hematocrit tube during a retro-orbital bleed can sever the optic nerve, resulting in blindness. If the hematocrit tube is advanced at an improper angle, the eye can be forced out of the orbit, allowing the eyelids to fall behind the eyeball. If this occurs, it is very difficult to correctly replace the eye into the socket. Other issues that can arise include fracturing of the fragile orbit bones, penetration of the eye globe that results in the loss of vitreous humour, or the formation of a hematoma behind the eye that can result in extreme pain due to the pressure on the eye and surrounding structures. Despite all of these concerns, if a skilled technician performs the procedure and the animal is fully anesthetized with a general anesthetic, such as isoflurane inhalant anesthesia, retro-orbital bleeding has been shown to be an effective method of blood collection in rodents.
The anatomical structure of the orbital area is different between the mouse and rat. The mouse has the retro-orbital sinus-a collection of vessels that create a sinus in the orbital area. In the orbit of the rat eye, there is a plexus of vessels that flow behind that eye; however, they do not form a sinus, as in the mouse. Consequently, it is easier to perform this procedure on mice. For repeated sampling collection via the retro-orbital plexus, a minimum of 10 days between bleeds is required to allow the tissues in the area to heal. Although general anesthesia is recommended, the procedure can be performed in mice without general anesthesia if a topical ophthalmic anesthetic, such as proparacaine or tetracaine, is applied prior to the procedure. As rats do not have the retro-orbital sinus, and because their membranes around the orbit are much stronger, it is mandatory to anesthetize them for this procedure.
Serial samples of a small volume can be obtained by using a tail clip method. The initial amputation of the tail must be limited to a tail tip, approximately 0.5-1.0 mm in length in mice and 2.0 mm in rats.1 The tail snip procedure for blood collection allows for serial collections by disrupting the scab or clot of the original cut at the end of the tail. Generally, additional amputation of the tail tip is not necessary. Volumes of blood collected range from 20-100 µL for mice and 75-150 µL for rats. The amount collected is variable between animals and can be influenced by age, health status, and weight.
The sample collected from a tail snip can contain both arterial and venous blood, along with tissue product contamination. The sample quality decreases if the tail is stroked or "milked" to obtain more blood. To increase blood flow, the tail can be heated with warm compresses, a heat lamp, or submersion in warm water. Pressure should be applied to the tail tip for hemostasis, and animals should be checked every 5-10 minutes to ensure hemostasis has been achieved. Hemostasis is often delayed with repeated sampling. A styptic powder may be used for hemostasis. For the initial amputation, anesthesia (general or local) is recommended. Subsequent bleeding should not require anesthesia, especially as the animals become habituated to the procedure. Anesthesia will cause a drop in blood pressure, making blood collection with this technique difficult.
An alternative to a tail snip is the tail vessel nick. This procedure is easily performed on both mice and rats. However, as with the tail snip, the samples may be contaminated with tissue products, especially in the mouse. For rats, a hypodermic needle is inserted into the vessel, and the blood is collected from the hub of the needle. One study demonstrated the use of a tourniquet placed above the needle puncture site to aid in blood collection.3 A syringe is not used to draw the blood out of the vessel, as the pressure created from the syringe will collapse the vessel. This method can also be used for serial sampling, as a clot can be removed to cause the site to bleed again. As with tail snips, it is imperative to ensure hemostasis by applying pressure to the site and rechecking the animal every 5-10 minutes.
Often, studies require a nonsurvival, large blood sample that is collected through exsanguination via an intracardiac bleed or the caudal vena cava.4 Approximately half of the total blood volume can be collected from a mouse or rat by cardiac puncture. This is equivalent to 40 µl/g or approximately 1 ml for an average 25 gram mouse. A 250 gram rat would yield approximately 10 ml of blood. The animal must be anesthetized for exsanguination. Inhalant anesthesia or CO2 narcosis can be used by a proficient technician; injectable anesthesia can also be used. However, there may be a decrease in blood pressure and circulation, which could decrease the amount of blood collected.
The caudal vena cava method requires that the animal be deeply anesthetized to surgically expose the vessel. CO2 narcosis is not sufficient, as the heart must be beating and the animal breathing during blood withdrawal. During the procedure, too rapid of blood withdrawal can cause the vessel to collapse onto the bevel of the syringe, occluding the opening and preventing blood collection. Also, the vessel walls are thin, and thus movement of the hand and needle must be avoided to prevent rupture or leaking of blood from the needle entry site. As the needle is not passing through the skin, this method results in the collection of a sterile sample. Adjunctive euthanasia methods must be employed to ensure that the animal does not recover from anesthesia. This method is often followed by cardiac or aortic perfusion.
The intracardiac method can be performed either with the animal restrained manually once it is anesthetized (closed method), or the heart can be surgically exposed as per the protocol for caudal vena cava blood collection method (open method). For the closed method, the landmarks for needle placement are the groove formed by the rib cage at the xiphoid process, on the animal's left side.
Procedure
1. Retro-orbital bleed
- Equipment
- Prepare a bell jar, or anesthetic induction chamber, to administer an anesthetic gas such as isoflurane. When using a bell jar, it is imperative that the liquid anesthetic does not come into contact with the animal, to avoid absorption through the skin. A platform with small holes can be used.
- Microhematocrit tubes that hold 50-75 microliters are preferred. Mylar wrapped tubes are less likely to break between the fingers of the operator and should be considered as a safety measure.
- Several paper towel thicknesses, or other insulating materials, are placed on the work surface to maintain the animal's body heat during the procedure.
- Preparation and positioning of the animal
- The animal is anesthetized with an inhalation anesthetic, such as isoflurane, in a bell jar or gas anesthesia induction chamber, to effect.
- Once the animal is fully anesthetized, it is removed and placed in lateral recumbency.
- The eye is protruded by placing a finger on the top of the head and along the jawline, and pulling the skin back and down.
- Avoid applying pressure to the trachea, as that may collapse or occlude the airway causing death by asphyxia.
- Blood withdrawal
- The microhematocrit is placed in the medial canthus of the eye and directed caudally at a 30-45° angle from the plane of the nose.
- Apply pressure while gently rotating the hematocrit tube. This will cut through the conjunctival membranes and rupture the ocular plexus.
- The blood will flow into the hematocrit tube by capillary action.
- Avoid pushing so deep that you hit the bone at the back of the ocular cavity.
- Once blood begins to flow, maintain pressure to keep the eye protruded.
- To collect multiple tubes of blood, it is not necessary to place the next tube into the ocular plexus, as the blood will continue to flow and can be collected as it comes from the medial canthus.
- To stop bleeding, release the skin and allow the eye to return to the normal position. Apply pressure to the orbit to ensure hemostasis.
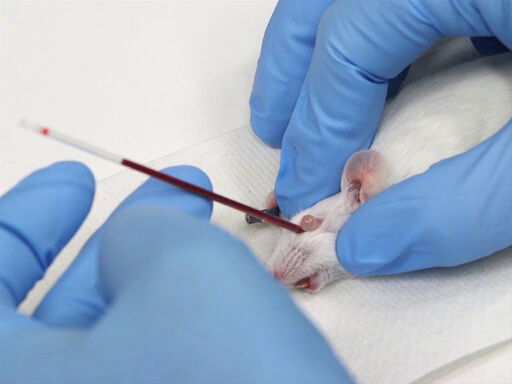
Figure 1. Retro orbital blood withdrawal in mice.
2. Tail bleed procedures: tail snip and tail nick
- Equipment
- A sterile scalpel blade, preferably a number 11 blade or a single-sided razor blade, is used to make the initial amputation for the tail snip method. Scissors should not be used because the cut made by scissors is crushing, thus promoting clotting and reducing blood flow. For the tail nick procedure, a number 11 or 15 scalpel blade is used to make the cut.
- A restraint tube that allows access to the tail of the mouse is prepared.
- Absorbent paper towels or gauze are used as the substrate for performing the tail snip.
- Collection tubes or hematocrit tubes are also required.
- Styptic powder should be available to aid in hemostasis.
- Restraint
- The animal is placed into the tube such that the tail is accessible. For Broome type restrainers, the animal is pulled rump first into the tube. For other tubes, the animal is placed head first.
- Animals are secured into the tube such that they cannot turn around or withdraw the tail.
- Some mice will allow the tail snip and blood collection with minimal manual restraint if they are allowed to grab a rough surface.
- Some rats will require inhalation anesthesia for this method of blood collection.
- Blood withdrawal
- The tail is wiped with warm water to remove debris and cause slight vasodilation. DO NOT use hot water.
- For the tail snip, the tail is extended, and the very end of the tail (0.5-1 mm for mice and up to 2 mm for rats) is cut with the scalpel blade.
- For the tail nick, the tail is extended, and a cut is made with the scalpel blade approximately 2/3 the distance from the rump, directly over the lateral tail vein.
- The tail can be stroked from rump to tip to encourage blood flow; however, this will decrease the quality of the sample.
- The blood is collected from the tip or nick using hematocrit tubes or allowed to drip into a collection vial.
3. Cardiac blood collection
- Equipment
- For a mouse, a 3 cc syringe with a 22-25 gauge x 1" needle is preferred. Smaller syringes do not have the same back pressure and can make blood withdrawal more difficult. Needles smaller than 25 gauge restrict the flow of blood, leading to increased clotting and damage to the blood cells. Needles shorter than 1" may not reach the level of the heart when approaching from the diaphragm.
- For a rat, a 10-12 cc syringe with an 18 gauge x 1.5" needle is preferred. Depending on the size of the rat, a smaller syringe may not hold the entire blood volume to be collected, and thus the syringe would have to be changed during the procedure. Needles smaller than 20 gauge restrict the flow of the blood, leading to increased clotting. Needles shorter than 1.5" may not reach the level of the heart when approaching from the diaphragm.
- A blood collection tube of sufficient size is used to hold the blood collected.
- Restraint
- Proper restraint is essential to the success of this method. The animal is held by the scruff with the body hanging vertically. It is important that the body be straight to prevent deflection of the heart or a twisting of the chest.
- An alternative position is dorsal recumbency when placing the needle between the ribs on the animal's left side. This is especially useful for very large rats or when multiple animals are to be bled.
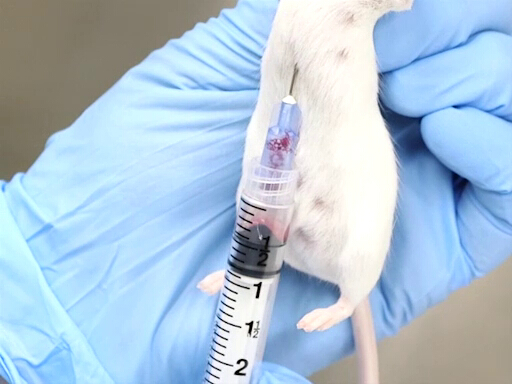
Figure 2. Cardiac blood withdrawal with mouse held vertically.
- Blood withdrawal
- The approach from the posterior aspect, puncturing the diaphragm is more easily accomplished when the mouse or rat is held vertically by the scruff.
- The needle is advanced in the notch just to the left of the animal's xiphoid.
- The needle should be parallel to the spine and placed just under the ribs.
- The heart is located approximately at the level of the elbow.
- Place the needle, bevel up, into the chest, and puncture the heart.
- Apply slight back pressure with the syringe. If the needle is in the heart, blood will flow into the syringe.
- Wait until blood has filled the syringe before adding additional back pressure on the syringe.
- The lateral approach from the animal's left side requires positioning the animal in dorsal recumbency.
- The point of entry is measured against the point of the elbow on the chest wall. The heart is located approximately at the level of the elbow.
- The needle is inserted perpendicular to the plane of the table at a point midway on the chest wall as measured dorsoventrally.
- Place the needle, bevel up, into the chest, and puncture the heart.
- Apply slight back pressure with the syringe. If the needle is in the heart, blood will flow into the syringe.
- Wait until blood has filled the syringe before adding additional backpressure on the syringe.
- The approach from the posterior aspect, puncturing the diaphragm is more easily accomplished when the mouse or rat is held vertically by the scruff.
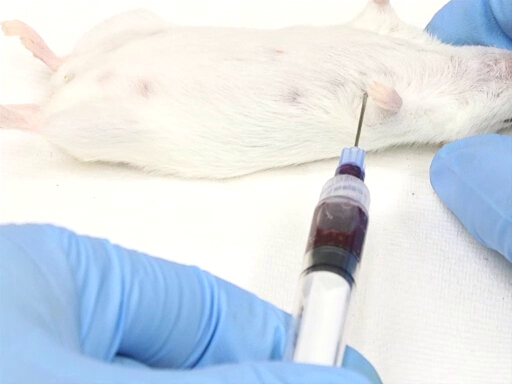
Figure 3. Cardiac blood withdrawal with mouse in dorsal recumbency position.
- Technical tips
- The normal heart is situated with the apex pointing to the left. In rare instances, the heart may be reversed, resulting in difficulty in puncturing the heart.
- Excessive back pressure on the syringe may collapse the heart, occluding the needle bevel and stopping blood flow into the syringe.
- Applying back pressure and releasing it repeatedly will initiate clotting in the syringe.
- Gently applying pressure to the liver can force additional blood volume into the circulatory system, making it available for withdrawal.
4. Posterior vena cava blood withdrawal
- Equipment
- A TB syringe with a 25-29 gauge needle is used for blood collection in the mouse. For rats, a 10-12 cc syringe with a 22-25 gauge x 1" needle is required.
- A surgical platform, dissection tray, or other surface to secure the animal is needed, along with ties, tape, or pins to affix the limbs in position.
- Injectable anesthesia or inhalation anesthesia is necessary. If using inhalation anesthesia, it is desirable that the anesthetic be delivered via a precision vaporizer with a nose cone. The procedure length is such that using an induction chamber without additional anesthetic gas delivery will not provide sufficient time to complete the blood withdrawal before the animal revives.
- Iris scissors for the mouse, or operating room sharp-blunt scissors for the rat, are required, along with small atraumatic thumb forceps, and a 2"x 2" gauze sponge.
- Restraint
- When the animal is fully anesthetized, as determined by toe pinch or tail pinch, the animal is placed in dorsal recumbency.
- The limbs are secured to the platform with tape or pins. The limbs should be extended away from the body.
- Withdrawal
- The skin is lifted and a small transverse cut is made through the skin just above the pelvis in females, or just above the prepuce in males.
- The point of the scissors is placed into the cut, and a midline incision is made through the skin from the pelvis/prepuce to the xiphoid.
- The skin is reflected laterally to each side. Blunt dissection may be necessary to loosen it from the underlying muscle.
- The muscle is lifted, and a small transverse cut is made through the muscle just above the skin cut.
- The point of the scissors is placed into the abdomen and a midline incision is made through the muscle to the xiphoid. Be sure to angle the scissors' point upward to avoid cutting any organs.
- Cut transversely along the curve of the ribs on each side. Exercise extra caution not to puncture the liver.
- Gently move the intestines to the animal's left to expose the posterior vena cava.
- Place a gauze pad on the liver, and rest the index and middle finger on the liver.
- With the other hand, insert the needle, bevel upward, into the vena cava midway between the junction of the renal vessels and the iliac bifurcation.
- Slowly withdraw the blood while applying pressure on the liver.
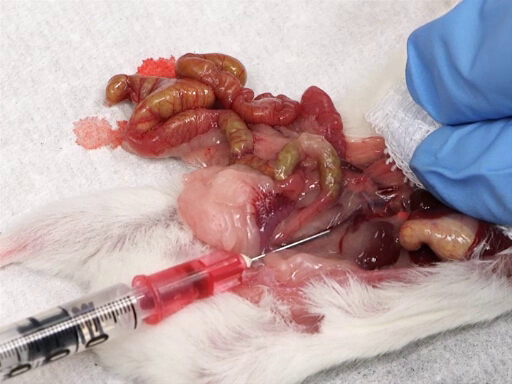
Figure 4. Blood withdrawal from posterior vena cava.
Application and Summary
Blood collection for mice and rats can be accomplished with a variety of techniques. Although many factors, such as sample size, frequency of sampling, and the size and age of the animal influence this, the most essential component is the skill level of the technician performing the sample collection. For the methods described here, the proper use of anesthetics is also crucial for quality samples and the wellbeing of the animals.
References
- Guidelines for the survival bleeding of mice and rats. 2010.
- Diehl, K.H., Hull, R., Morton, D., Pfister, R., Rabemampianina, Y., Smith, D., Vidal, J.M., and van de Vorstenbosch, C. 2001. A good practical guide to the administration of substances and removal of blood, including routes and volumes. Journal of Applied Toxicology. 21. 15-23.
- Omaye, S.T., Skala, J.H., Gretz, M.D., Schaus, E.E., and Wade, C.E. 1987. Simple method for bleeding the unanaesthetized rat by tail venipuncture. Laboratory Animals. 21. 261-264.
- Adeghe, A.J-H. and Cohen, J. 1986. A better method for terminal bleeding of mice. Laboratory Animals. 20. 70-72.
Tags
Skip to...
Videos from this collection:

Now Playing
Blood Withdrawal I
Lab Animal Research
171.7K Views

Rodent Handling and Restraint Techniques
Lab Animal Research
174.5K Views

Basic Care Procedures
Lab Animal Research
28.0K Views

Fundamentals of Breeding and Weaning
Lab Animal Research
35.7K Views

Rodent Identification I
Lab Animal Research
54.8K Views

Rodent Identification II
Lab Animal Research
25.6K Views

Compound Administration I
Lab Animal Research
100.6K Views

Compound Administration II
Lab Animal Research
34.9K Views

Compound Administration III
Lab Animal Research
31.5K Views

Compound Administration IV
Lab Animal Research
51.7K Views

Blood Withdrawal II
Lab Animal Research
73.2K Views

Anesthesia Induction and Maintenance
Lab Animal Research
50.5K Views

Considerations for Rodent Surgery
Lab Animal Research
22.5K Views

Diagnostic Necropsy and Tissue Harvest
Lab Animal Research
58.1K Views

Sterile Tissue Harvest
Lab Animal Research
34.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved