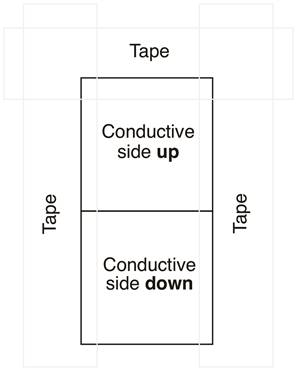
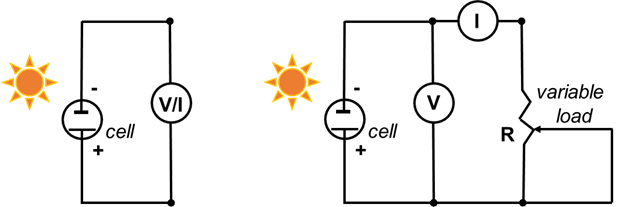
Dye-sensitized Solar Cells
Overview
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
Today's modern world requires the use of a large amount of energy. While we harness energy from fossil fuels such as coal and oil, these sources are nonrenewable and thus the supply is limited. To maintain our global lifestyle, we must extract energy from renewable sources. The most promising renewable source, in terms of abundance, is the sun, which provides us with more than enough solar energy to fully fuel our planet many times over.
So how do we extract energy from the sun? Nature was the first to figure it out: photosynthesis is the process whereby plants convert water and carbon dioxide to carbohydrates and oxygen. This process occurs in the leaves of plants, and relies on the chlorophyll pigments that color the leaves green. It is these colored molecules that absorb the energy from sunlight, and this absorbed energy which drives the chemical reactions.
In 1839, Edmond Becquerel, then a 19-year old French physicist experimenting in his father's lab, created the first photovoltaic cell. He illuminated an acidic solution of silver chloride that was connected to platinum electrodes which generated a voltage and current.1 Many discoveries and advances were made in the late 19th and first half the 20th century, and it was only in 1954 that the first practical solar cell was built by Bell Laboratories. Starting in the 1950s, solar cells were used to power satellites in space.2
Solar cells are electrical devices that utilize light to create a current. This video demonstrates preparation and testing of one such type of cell, the dye-sensitized solar cell (DSSC). First invented at UC Berkeley by Brian O'Regan and Michael Grätzel, Grätzel pursued this work at the École Polytechnique Fédérale de Lausanne in Switzerland, culminating in the first highly efficient DSSC in 1991.3 These solar cells, like plants, use a dye to help harness energy from the sun.
Procedure
1. Preparation of TiO2 Paste
- Mass out 6 g of colloidal TiO2 powder, and place it in a mortar.
- Carefully add 2-3 mL of vinegar to the TiO2, and begin grinding the suspension with the pestle until a uniform paste is obtained. The grinding serves to break up aggregated clumps in the powder.
- Continue adding vinegar, in ~ 1 mL increments while grinding, up to ~ 9 mL total volume. Prior to each addition, the consistency of the paste should be uniform and free of
Results
For each data point collected in steps 6.5.3-6.5.4, calculate the current density (mA/cm2) and the power density (mW/cm2). To calculate the current density, divide the current by the surface area of the film that was determined in step 2.7. To calculate the power density, multiply the voltage by current density. Plot the current (mA) versus voltage (mV) for the data collected in steps 6.3, 6.4, and 6.5.3-6.5.4. Plot the current density versus volts for all the data.
Application and Summary
This video showed the preparation and analysis of a simple DSSC.
Solar cells are becoming more common, and there is much research being done to advance their performances. Traditional solar cells that are based on silicon semiconductors are used to make solar panels that are used in space and on earth. The Denver International Airport makes use of Colorado's sunny climate and has four solar arrays which provides 6% of the airport's energy needs.
Log in or to access full content. Learn more about your institution’s access to JoVE content here
References
- Williams, R. Becquerel Photovoltaic Effect in Binary Compounds. J Chem Phys, 32 (5), 1505-1514 (1960).
- Perlin (2005), Late 1950s - Saved by the Space Race", Solar Evolution - The history of Solar Energy. The Rahus Institute. Retrieved 28 June 2016.
- Regan, B., Gratzel, M. Nature, 353, 737-740 (1991).
- Miessler, G. L., Fischer, P. J., Tarr, D. A. Inorganic Chemistry, Pearson, 2014.
- Wikipedia page: Dye-sensitized solar cell,
- Smestad, G. P., Grätzel, M. Demonstrating Electron Transfer and Nanotechnology: A Natural Dye-Sensitized Nanocrystalline Energy Converter. J Chem Ed. 75 (6), 752 (1998).
- Burschka, J., Pellet, N., Moon, S.-J., Humphry-Baker, R., Nazeeruddin, M. K., Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature, 499 (7458), 316-9 (2013).
Tags
Skip to...
Videos from this collection:

Now Playing
Dye-sensitized Solar Cells
Inorganic Chemistry
15.7K Views

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.5K Views

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Views

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.3K Views

The Evans Method
Inorganic Chemistry
68.0K Views

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
104.0K Views

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.4K Views

Mössbauer Spectroscopy
Inorganic Chemistry
21.9K Views

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.7K Views

Structure Of Ferrocene
Inorganic Chemistry
79.1K Views

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
45.0K Views

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.1K Views

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Views

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.5K Views

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.7K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved