Quadruply Metal-Metal Bonded Paddlewheels
Overview
Source: Corey Burns, Tamara M. Powers, Department of Chemistry, Texas A&M University
Paddlewheel complexes are a class of compounds comprised of two metal ions (1st, 2nd, or 3rd row transition metals) held in proximity by four bridging ligands (most commonly formamidinates or carboxylates) (Figure 1). Varying the identity of the metal ion and the bridging ligand provides access to large families of paddlewheel complexes. The structure of paddlewheel complexes allows for metal-metal bonding, which plays a vital role in the structure and reactivity of these complexes. Due to the diversity of electronic structures that are available to paddlewheel complexes - and the corresponding differences in M-M bonding displayed by these structures - paddlewheel complexes have found application in diverse areas, such as in homogeneous catalysis and as building blocks for metal-organic frameworks (MOFs). Understanding the electronic structure of the M-M bonds in paddlewheel complexes is critical to understanding their structures and thus to application of these complexes in coordination chemistry and catalysis.
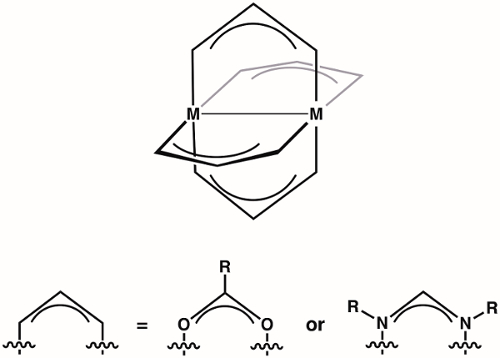
Figure 1. General structure of paddlewheel complexes, where M can be a 1st, 2nd, or 3rd row transition metal.
When two transition metals are held in close proximity the d-orbitals overlap, which can result in the formation of M-M bonds. Overlapping d-orbitals can form three types of bonds - σ, π, and δ - depending on the symmetry of the orbitals involved. If we assign the molecular z-axis to be coplanar with the M-M bond, a σ bond is formed by overlap of the dz2 orbitals and π bonds are formed by overlap of the dxz and dyz orbitals. δ bonds are generated by overlap of d-orbitals that have two planar nodes (dxy and dx2–y2). As a result, all four lobes of the d-orbital overlap and the corresponding δ bond has two planar nodes (Figure 2). In theory, with the addition of δ bonds, paddlewheel complexes are capable of supporting quintuple bonds, or five bonds between metal atoms.1 In most complexes, the dx2–y2 forms strong metal-ligand bonds and does not meaningfully contribute to M-M bonding. Thus, quadruple bonds are the maximum bond order in many complexes.
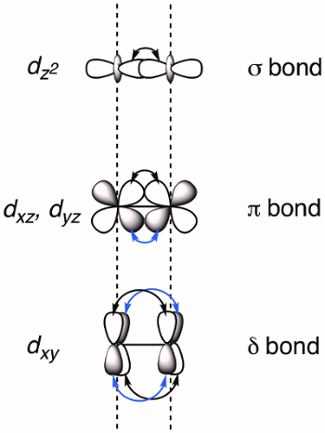
Figure 2. Visual representation of σ, π, and δ bonding MOs resulting from the linear combination of metal d-orbitals. The dz2 atomic orbitals have the best spatial overlap, followed by the dxz and dyz orbitals. The dxy atomic orbitals have the least amount of spatial overlap.
In this video, we will synthesize the dimolybdenum paddlewheel complex Mo2(ArNC(H)NAr)4, where Ar = p-(MeO)C6H4, which features a quadruple bond. We will characterize the compound by NMR spectroscopy and use X-ray crystallography to study the M-M bond.
Procedure
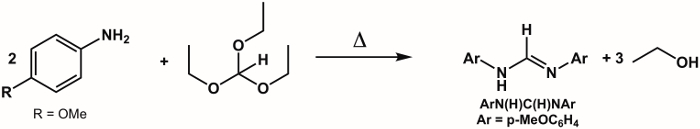
1. Synthesis of Ligand ArN(H)C(H)NAr, Where Ar = p-(MeO)C6H4 (Figure 5)2
- Combine 6.0 g (0.050 mol) of p-anisidine and 4.2 mL (0.025 mol) of triethylorthoformate in a 100 mL round bottom flask with a magnetic stir bar.
- Attach a distillation head to the reaction flask.
- With stirring, heat the reaction in an oil bath to reflux (120 °C). Once reflux is achieved, the byproduct ethanol should begin
Results
Ligand ArN(H)C(H)NAr
Yield: 3.25 g (53%). 1H NMR (chloroform-d, 500 MHz, δ, ppm): 8.06 (s, 1H, NHC-HN), 6.99 (d, 4H, aromatic C-H, J = 8.7 Hz), 6.86 (d, 4H, aromatic C-H, J = 9.0 Hz), 3.80 (s, 6H, -OCH3).
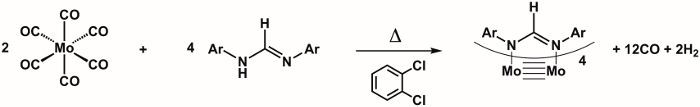
Mo complex Mo2(ArNC(H)NAr)4
Yield: 450 mg (57%). 1
Application and Summary
In this video, we learned about M-M bonding. We synthesized a dinuclear molybdenum complex featuring a quadruple bond. Quadruple bonds consist of three different bond types, including σ, π, and δ bonds. We collected single crystal X-ray diffraction data and observed a short Mo-Mo bond length consistent with a quadruply bonded compound.
Paddlewheel complexes, such as the Mo2 complex prepared here, display a wide range of properties and thus find application in diverse
References
- Nguyen, T., Sutton, A. D., Brynda, M., Fettinger, J. C., Long, G. J., Power, P. P. Synthesis of a stable compound with fivefold bonding between two chromium(I) centers. Science. 310(5749), 844-847 (2005).
- Lin, C., Protasiewicz, J. D., Smith, E. T., Ren, T. Linear free energy relationship in dinuclear compounds. 2. Inductive redox tuning via remote substituents in quadruply bonded dimolybdenum compounds. Inorg Chem. 35(22), 6422-6428 (1996).
- Cotton, F. A., Murillo, C. A., Walton, R. A. Eds. Multiple Bonds Between Metal Atoms, 3rd ed. Springer. New York, NY. (2005).
- Nakamura, E., Yoshikai, N., Yamanaka, M. Mechanism of C−H Bond Activation/C−C Bond Formation Reaction between Diazo Compound and Alkane Catalyzed by Dirhodium Tetracarboxylate. J Am Chem Soc. 124 (24), 7181-7192 (2002).
Skip to...
Videos from this collection:

Now Playing
Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Views

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.6K Views

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Views

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.7K Views

The Evans Method
Inorganic Chemistry
68.6K Views

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
104.7K Views

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.5K Views

Mössbauer Spectroscopy
Inorganic Chemistry
22.0K Views

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.9K Views

Structure Of Ferrocene
Inorganic Chemistry
79.6K Views

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
45.5K Views

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.4K Views

Dye-sensitized Solar Cells
Inorganic Chemistry
15.8K Views

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.7K Views

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved