Application of Group Theory to IR Spectroscopy
Overview
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
Metal carbonyl complexes are used as metal precursors for the synthesis of organometallic complexes as well as catalysts. Infrared (IR) spectroscopy is one of the most utilized and informative characterization methods of CO containing compounds. Group theory, or the use of mathematics to describe the symmetry of a molecule, provides a method to predict the number of IR active C-O vibrational modes within a molecule. Experimentally observing the number of C-O stretches in the IR is a direct method to establish the geometry and structure of the metal carbonyl complex.
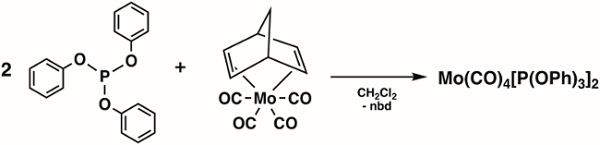
In this video, we will synthesize the molybdenum carbonyl complex Mo(CO)4[P(OPh)3]2, which can exist in the cis- and trans-forms (Figure 1). We will use group theory and IR spectroscopy to determine which isomer is isolated.

Figure 1. The cis- and trans-isomers of Mo(CO)4[P(OPh)3]2.
Procedure
1. Setup of the Schlenk Line (for a more detailed procedure, please review the "Schlenk Lines Transfer of Solvent" video in the Essentials of Organic Chemistry series). Schlenk line safety should be reviewed prior to conducting this experiment. Glassware should be inspected for star cracks before use. Care should be taken to ensure that O2 is not condensed in the Schlenk line trap if using liquid N2. At liquid N2 temperature, O2 condenses and is
Results
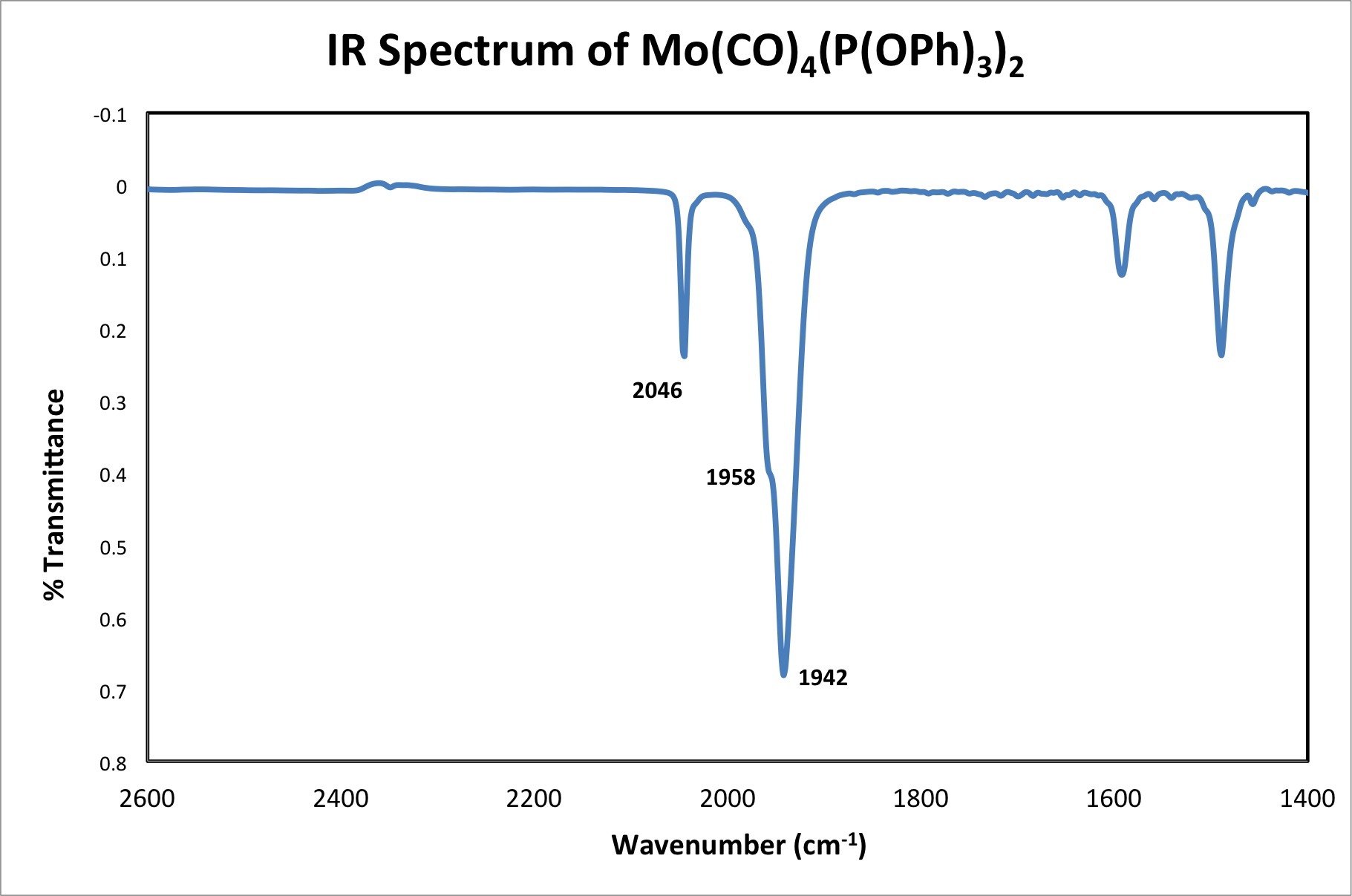
Figure 5. IR of Mo(CO)4[P(OPh)3]2.
Solution IR in saturated hydrocarbon (cm-1): 2046 (s), 1958 (s), 1942 (vs).
The fourth resonance can only be seen under high-resolution conditions. Therefore, it is possible, as in this case, that only 3 of the 4 resonances are observed.
Based on the obtained IR, we can conclu
Application and Summary
In this video, we learned how to use group theory to predict the number of IR active vibrational modes in a molecule. We synthesized the molecule Mo(CO)4[P(OPh)3]2 and used IR to determine which isomer was isolated. We observed that the product had three C-O vibrations in its IR spectrum, which is consistent with the cis-isomer.
Group theory is a powerful tool that is used by chemists to not only predict IR active vibrational modes, but also vibrationa
References
- Fukumoto, K., Nakazawa, H. Geometrical isomerization of fac/mer-Mo(CO)3(phosphite)3 and cis/trans-Mo(CO)4(phosphite)2 catalyzed by Me3SiOSO2CF3. J Organomet Chem. 693(11), 1968-1974 (2008).
- Darensbourg, M. Y., Magdalena, P., Houliston, S. A., Kidwell, K. P., Spencer, D., Chojnacki, S. S., Reibenspies, J. H. Stereochemical nonrigidity in heterobimetallic complexes containing the bent metallocene-thiolate fragment. Inorg Chem. 31(8), 1487-1493 (1992).
- Darensbourg, M. Y., Darensbourg, D. J. Infrared Determination of Stereochemistry in Metal Complexes. J Chem Ed. 47(1), 33-35 (1970).
Skip to...
Videos from this collection:

Now Playing
Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
45.3K Views

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.6K Views

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Views

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.7K Views

The Evans Method
Inorganic Chemistry
68.6K Views

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
104.6K Views

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.5K Views

Mössbauer Spectroscopy
Inorganic Chemistry
22.0K Views

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.9K Views

Structure Of Ferrocene
Inorganic Chemistry
79.6K Views

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.4K Views

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Views

Dye-sensitized Solar Cells
Inorganic Chemistry
15.8K Views

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.7K Views

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved