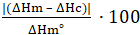Differential Scanning Calorimetry
Overview
Source: Danielle N. Beatty and Taylor D. Sparks, Department of Materials Science and Engineering, The University of Utah, Salt Lake City, UT
Differential scanning calorimetry (DSC) is an important measurement to characterize thermal properties of materials. DSC is used primarily to calculate the amount of heat stored in a material as it heats up (heat capacity) as well as the heat absorbed or released during chemical reactions or phase changes. However, measurement of this heat can also lead to the calculation of other important properties such as glassy transition temperature, polymer crystallinity, and more.
Due to the long, chain-like nature of polymers it is not uncommon for polymer strands to be entangled and disordered. As a result, most polymers are only partially crystalline with the remainder of the polymer being amorphous. In this experiment we will utilize DSC to determine polymer crystallinity.
Procedure
- Turn on the machine and allow it to warm up for about an hour.
- Check to ensure the compressed nitrogen tank and liquid nitrogen tank are both full and the valve connecting them is open. The compressed nitrogen pressure flow is set at 10 psi by the adjustment knobs on the regulator.
- Prepare two empty pans. Poke a small hole in the lid of each and seal by using the crimping press. Remove the three furnace lids and place the pans on the two circular sensors within the furnace. Replace all three lids.
- Click o
Results
Figure 3 shows the result of a DSC percent crystallinity sample scan on a polybutylene terephthalate (PBT) polymer sample. The result is displayed as a DSC power reading (in milliwatts per milligram of sample) verses time. The power reading, the blue trace in Figure 3, indicates how much additional power was required to change the temperature of the sample pan in comparison to the empty reference pan. The temperature program is a
Application and Summary
Differential scanning calorimetry is a technique used to determine many thermal properties of materials, such as heat of melting, heat of crystallization, heat capacity, and phase changes. DSC measurements can also be used to calculate additional material properties including glassy transition temperature and polymer percent crystallinity. The DSC requires very small samples that must conform to the size and shape of the pans used in the machine and is based on a differential heat comparison between an empty reference an
Skip to...
Videos from this collection:

Now Playing
Differential Scanning Calorimetry
Materials Engineering
37.4K Views

Optical Materialography Part 1: Sample Preparation
Materials Engineering
15.4K Views

Optical Materialography Part 2: Image Analysis
Materials Engineering
11.0K Views

X-ray Photoelectron Spectroscopy
Materials Engineering
21.5K Views

X-ray Diffraction
Materials Engineering
88.8K Views

Focused Ion Beams
Materials Engineering
8.8K Views

Directional Solidification and Phase Stabilization
Materials Engineering
6.5K Views

Thermal Diffusivity and the Laser Flash Method
Materials Engineering
13.2K Views

Electroplating of Thin Films
Materials Engineering
20.0K Views

Analysis of Thermal Expansion via Dilatometry
Materials Engineering
15.7K Views

Electrochemical Impedance Spectroscopy
Materials Engineering
23.1K Views

Ceramic-matrix Composite Materials and Their Bending Properties
Materials Engineering
8.1K Views

Nanocrystalline Alloys and Nano-grain Size Stability
Materials Engineering
5.1K Views

Hydrogel Synthesis
Materials Engineering
23.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved