Electrochemical Measurements of Supported Catalysts Using a Potentiostat/Galvanostat
Overview
Source: Laboratory of Dr. Yuriy Román — Massachusetts Institute of Technology
A potentiostat/galvanostat (often referred to as simply a potentiostat) is an instrument that measures current at an applied potential (potentiostatic operation) or measures potential at an applied current (galvanostatic operation) (Figure 1). It is the most commonly used instrument in the electrochemical characterization of anode and cathode materials for fuel cells, electrolyzers, batteries, and supercapacitors.
Conventionally, these anode and cathode materials are interfaced with a potentiostat via a three-electrode electrochemical cell. The electrode leads from the potentiostat are connected to the reference electrode, the counter electrode (often called the auxiliary electrode), and the working electrode (which contains the test material of interest). The electrochemical cell is then filled with a high ionic strength electrolyte solution, such as an acidic, alkaline, or salt solution. The media for this high ionic strength solution is typically aqueous; however, for applications necessitating higher operating cell potential windows, such as batteries and supercapacitors, non-aqueous media is often used. The cell media is degassed with an inert gas (to prevent unwanted side reactions) or with a test gas (if the test reaction involves a gas at one of the electrodes).
Alternatively, a salt bridge or membrane is employed to maintain ionic contact if the two half cells are to be measured in different electrolytes. In heterogeneous electrocatalysis, this type of "two compartment" cell is often used if the test molecule at the working electrode is also reactive at the counterelectrode. This happens frequently as the counterelectrode typically employed is platinum, which is a highly active catalyst for many reactions. Here, single compartment cells will be used, where all three electrodes are in the same media.
This video will explain the process of polishing a working electrode, preparing a catalyst ink, mounting the catalyst ink onto the working electrode, preparing the electrochemical cell, and then performing electrochemical measurements. The measurements that are performed include: cyclic voltammetry (CV), linear sweep voltammetry (LSV), chronopotentiometry (CP), and chronoamperometry (CA).
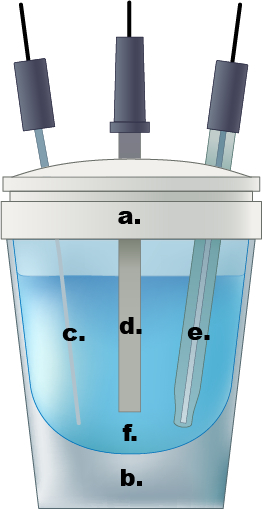
Figure 1. An example of a single compartment electrochemical cell. a.) Teflon cap, b.) glass cell, c.) Pt wire counter electrode, d.) working electrode, e.) Ag/AgCl reference electrode, f.) 0.5 M aqueous sulfuric acid electrolyte solution.
Procedure
1. Catalyst Ink and Working Electrode Preparation
Safety Precautions: Metals supported on carbon black must be handled in a fume hood or balance enclosure until it is in suspension form as these powders are inhalation hazards.
- Using an enclosed balance, weigh out 5–10 mg of metal/carbon black catalyst and add to a glass vial with a cap.
- Using a micropipette, dilute the catalyst with water such that the final concentration is 7.5 mg of catalyst per m
Results
This procedure will result in figures containing plots of measured current vs. potential for each of the four techniques. By convention for CV and LSV, the plots will also be outputted as measured current vs. potential despite the reality that these are transient techniques that measure current vs. the time derivative of potential.
Application and Summary
CV, LSV, CP, and CA are indispensable techniques for determining the efficacy of new electrode materials for fuel cells, electrolyzers, batteries, and supercapacitors as well as for developing fields such as the selective partial oxidation or reduction of commodity chemicals. These methods allow for determining overpotentials of reactions on different electrode materials as compared to their thermodynamic equilibrium potentials. These methods also allow the volumetric or gravimetric capacitance of supercapacitors to be d
Skip to...
Videos from this collection:

Now Playing
Electrochemical Measurements of Supported Catalysts Using a Potentiostat/Galvanostat
Analytical Chemistry
51.3K Views

Sample Preparation for Analytical Characterization
Analytical Chemistry
84.5K Views

Internal Standards
Analytical Chemistry
204.6K Views

Method of Standard Addition
Analytical Chemistry
319.7K Views

Calibration Curves
Analytical Chemistry
795.9K Views

Ultraviolet-Visible (UV-Vis) Spectroscopy
Analytical Chemistry
622.8K Views

Raman Spectroscopy for Chemical Analysis
Analytical Chemistry
51.1K Views

X-ray Fluorescence (XRF)
Analytical Chemistry
25.4K Views

Gas Chromatography (GC) with Flame-Ionization Detection
Analytical Chemistry
281.6K Views

High-Performance Liquid Chromatography (HPLC)
Analytical Chemistry
383.9K Views

Ion-Exchange Chromatography
Analytical Chemistry
264.3K Views

Capillary Electrophoresis (CE)
Analytical Chemistry
93.6K Views

Introduction to Mass Spectrometry
Analytical Chemistry
112.1K Views

Scanning Electron Microscopy (SEM)
Analytical Chemistry
87.0K Views

Cyclic Voltammetry (CV)
Analytical Chemistry
124.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved