需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
的额边缘活度测量在儿童使用情绪穿着或行为古怪的任务与家族性高风险的精神分裂症
摘要
This paper describes how to use the emotional oddball task and fMRI to measure brain activation in children and adolescents at familial high risk for schizophrenia (FHR). FMRI was used to measure differences in fronto-striato-limbic regions during an emotional oddball task. Children with FHR exhibited abnormal functional activation during adolescence.
摘要
Adolescence is a critical developmental period where the early symptoms of schizophrenia frequently emerge. First-degree relatives of people with schizophrenia who are at familial high risk (FHR) may show similar cognitive and emotional changes. However, the neurological changes underlying the emergence of these symptoms remain unclear. This study sought to identify differences in frontal, striatal, and limbic regions in children and adolescents with FHR using functional magnetic resonance imaging. Groups of 21 children and adolescents at FHR and 21 healthy controls completed an emotional oddball task that relied on selective attention and the suppression of task-irrelevant emotional information. The standard oddball task was modified to include aversive and neutral distractors in order to examine potential group differences in both emotional and executive processing. This task was designed specifically to allow for children and adolescents to complete by keeping the difficulty and emotional image content age-appropriate. Furthermore, we demonstrate a technique for suitable fMRI registration for children and adolescent participants. This paradigm may also be applied in future studies to measure changes in neural activity in other populations with hypothesized developmental changes in executive and emotional processing.
引言
Schizophrenia is a neurodevelopmental disorder with a known genetic component1,2 and with symptoms including deficits in both executive and emotional processing3,4. First-degree relatives are thought to be at an increased risk of developing schizophrenia, and have been shown to share some of these same neurocognitive deficits in both cognitive and social-emotional domains5. We therefore expect that brain activity in regions associated with executive and emotional processing may be altered in at-risk family members preceding the onset of clinical symptoms.
Previous studies have indicated that both adults with schizophrenia and adults at familial high risk show aberrant activity within executive and emotional processing networks; however it remains unclear how these changes come about during development. Demonstrating that these changes occur early in life will be a critical first step in understanding the pathophysiology of the disorder. Therefore, this study utilizes an emotional oddball paradigm during functional MRI (fMRI) scanning in order to measure brain activity during the completion of a task that requires both executive and emotional processing in adolescents who are at risk for developing schizophrenia. Oddball paradigms are frequently used to examine the function of fronto-striate circuitry in schizophrenia6 and in individuals with familial high risk7 by measuring selective attention processes allocated to task-relevant target stimuli. Here, a standard oddball task has been modified to include task-irrelevant aversive and neutral stimuli that have been shown to elicit changes in brain activity in patients with schizophrenia8.
This paper measures functional differences between healthy adolescents and adolescents at high familial risk for schizophrenia using an emotional oddball task. The task design is similar to that used by Fichtenholtz and colleagues9, but the selection of aversive emotional images has been modified to be appropriate for children between the ages of 9-18. The use of this task during functional MRI allowed for the identification of specific brain regions that showed patterns of hyperactivation and hypoactivation in children and adolescents with FHR for schizophrenia, in addition to age-related changes in neural activity during adolescent development.
Access restricted. Please log in or start a trial to view this content.
研究方案
在这项研究中所使用的研究技术被批准的机构审查委员会杜克大学(IRB)和北卡罗来纳大学 - 教堂山分校。
1.成像任务设计
- 生成呈现稀少目标刺激(圆)的更频繁标准刺激(加扰图像)的序列内的基于事件的行为的任务。的任务的示意图示于图1。出示使用CIGAL软件10的任务。
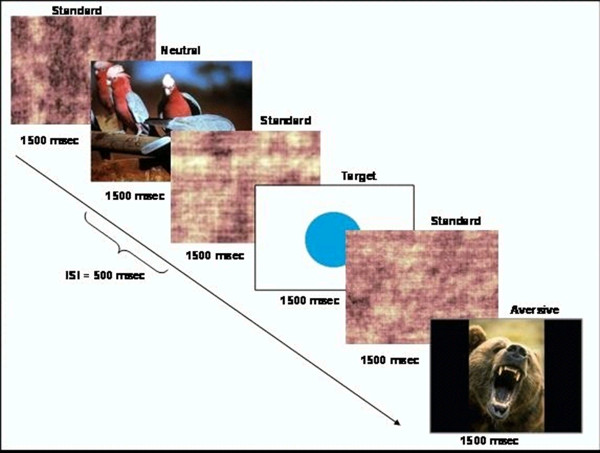
图1.原理任务设计的,这个数字已经被修改哈特等人 20,许可。 请点击此处查看该图的放大版本。
- 选择作为厌恶刺激等和一组从国际情感图片系统数据库(IAPS)中性刺激的。 IAPS图像以反映觉醒和价11的水平在额定1-9。高数值表示较高正价和觉醒。选择一组是年龄相适应的研究组,如蛇,蜘蛛,或其他动物的照片图像。
注意:用于此研究的任务无关的厌恶刺激图像具有3.38(标准差= 1.78)和6.14(标准差= 2.08)的平均觉醒评分的平均价的评价。中性刺激的图像有6.21(SD = 0.26)和3.72(SD = 2.15)的平均觉醒等级的平均价。 - 计划任务脚本,使得图像显示在一个伪随机的顺序为1500毫秒用500毫秒的平均刺激间的时间间隔。现在的目标刺激和任务无关的中性影像不超过频繁每15秒,使每个约4%的刺激。吉tter事件发生时间,以提供更好的分辨率的血流动力学响应函数。
- 创建8套图像,一个用于每个的8个功能的运行,使得与会者都带有总共40个目标和40的任务无关的中性图像对所有8次操作过程。
2.参与者设置和扫描
- 招募儿童和青少年的9岁至18岁谁不是健康对照个人或谁在家族性高风险的精神病之间。
- 确保健康的人没有精神疾病或任何一级的家庭成员有精神疾病。确保家族性风险的参与者都至少有一个一级亲属(父母或兄弟姐妹)患有精神分裂症。不排除他们对其他精神疾病的一级亲属的存在。
- 年龄和性别匹配的健康参与者家族危组参与者秒。
- 收购参与者知情同意18岁的未成年人超过,获取家长/监护人知情同意书。此外,收购未成年人参加学习谁写的同意。
- 将在一个模拟的核磁共振成像扫描仪的参与者,以便与环境的熟悉他们。播放扫描仪噪音的录音,让他们完成任务的行为的实践运行,以确保他们了解任务的说明。
- 将在核磁共振成像扫描仪的参与者,并获得必要的脑部扫描定位和/或解剖图像。
- 使用MRI安全的输入框,告诉参与者按下一个按钮,与他们的食指针对所有目标刺激和另一个按钮与中指所有其他刺激。
- 随着功能磁共振成像扫描,收集觉醒和价的参与者的一个子集,在研究中使用的图像的主观评价。该柯伦来自15个控制器和13家族高危T研究获得的评级。
3.图像采集
- 将参与者分为一个3.0特斯拉的核磁共振成像扫描仪。首先,获取了一组结构图像,包括一个3D共面解剖T1对比度图像使用一个被宠坏的梯度召回收购脉冲序列(TR的:5.16毫秒; TE:2.04毫秒,FOV:24厘米;图像矩阵:256×256;翻转角度:20;像素大小:0.94毫米×0.94毫米×1.9毫米; 68轴位片)。
- 采用梯度回波平面回波成像序列全脑覆盖采集功能成像数据(TR:2000毫秒; TE:27毫秒; FOV:24厘米;图像矩阵:64×64;翻转角度:60;像素大小:3.75毫米×3.75毫米×3.8毫米; 34轴位片),使大脑的活动可以在行为任务的执行过程中进行测量。竞选行为的任务的每次运行该成像序列。每次运行应包括120成像时间点。
- 目前TASK在8个功能运行时,每次持续约4分钟。
4.分析
- 图像预处理:开放式磁共振成像专家分析工具(技艺)的FSL 12。选择第一个层次的分析和预统计。
- 在 “数据”选项卡中,选择输入图像的数量和进入路径每次你要处理的MR图像。设置输出目录。进入总体积,废旧收购的数量,以及TR。
- 在“预统计 ”选项卡上,设置动态校正MCFLIRT,空间平滑FWHM为5彩信, 和 “ 切片定时校正”,选择“下注大脑提取”和“高通 ”时间滤波,但不要选择B0解弯曲(UNESS你有一个梯度场地图)或“我ntensitiy normaization”。12,14。
- 在 “ 注册”选项卡, 选择 “主STRUctural图像“。输入路径主体的头骨剥离T1加权像,用线性正常的搜索与至少6个自由度,选择的标准空间复选框,输入路径MNI图谱的图像,使用正常,线性搜索与12 DOF。 按 GO。
- 排除参与者大于3毫米头部运动在X,Y或Z方向。
- 1级:在一次运行中的任务条件之间的数据进行比较。开放式的壮举。选择“第一级分析”和 “ 统计+后统计”。
- 在数据选项卡,设置的输入数量,并输入路径到每个MR图像。输入“输出目录”的路径。 进入 “ 总卷”,放弃收购的数量,以及TR。
- 在“ 统计信息 ”选项卡,选择“ 使用针对电影预先白化 ”复选框16。按“全模式本身TUP“按钮。设置为任务条件的数量“原电动车的数量”。对于每个条件,“从双伽马HRF”卷积 “下拉菜单中选择17,18从基本形状下拉菜单和”自定义(3列格式 )“,并选择包含任务进度的文本文件。
- 格式化在3列这个文本文件与对于给定类型的每个“事件”一个条目。第一列应包含的发作时间(以秒计),第二应包含的持续时间(以秒为单位),以及第三应包含该事件的重量。在对比与F检验标签,创建一个对比每个任务的条件,并为每个比较。
- 在“后统计”选项卡中, 选择 “ 集群”中的“ 阈值”下拉菜单中,并设置“Z门槛”和群P牛逼hreshold为2.3和0.05分别为8,19。
- 在 “ 注册”选项卡, 选择 “主结构性形象”,输入路径主体的头骨剥离T1加权像。使用线性正常的搜索与至少6个自由度。 选择 “ 标准空间”复选框。输入路径MNI图谱形象。使用正常的,线性的自由度12搜索。按“开始”。
- 2级:比较运行之间的数据为每个任务的条件。开放式的壮举。选择从下拉菜单中选择“更高层次的分析”和“统计+后统计”。
- 在数据选项卡, 选择 “ 输入是低级别FEAT目录 ”。设置的输入的数量和输入路径到每个MR图像。输入“输出目录”的路径。
- 在 “ 统计信息”选项卡,将“ 混合效应:FLAME1”选择框“固定êffects“,按”模式设置向导“按钮,选择”单组平均“,然后单击”进程“按钮 。
- 在“后统计 ”选项卡中, 选择 “ 集群”中的“ 阈值 ”下拉菜单中,并设置“Z 门槛 ”和“ 群P”门槛为2.3和0.05分别为8,19。按“开始”。
- 3级:比较对象之间的数据在所有运行的每个任务的条件。开放式的壮举。选择从下拉菜单中选择“ 更高层次的分析 ”和“ 统计+后统计 ”。
- 在数据选项卡,选择“ 输入是3D从FEAT目录应对图像。”设置的输入数量,并输入路径到每个MR图像。输入“输出目录 ”的路径。
- 在 “ 统计”TAB,按“全模式的设置”。设置电动汽车等于组变量和协变量如诊断组,年龄,性别等输入每个受试者的值(输入1 - 输入n)的数目的数目为每个EV。您可以使用“粘贴”窗口,以复制这些值的电子表格。
- 在 “ 对比与F-测试 ”选项卡中,为每个测试变量和每个对比差异(例如,诊断组)添加对比。对于每个测试变量,设定由下的相应的EV选择该列中的值1的对比度。对于每一个相对,设置第一值1和第二为-1。选择“完成”。
- 在“后统计 ”选项卡中, 选择 “ 集群”中的“阈值”下拉菜单中,并设置“Z 门槛 ”和 “ 群P”门槛为2.3和0.05分别为8,19 。按“开始”。
Access restricted. Please log in or start a trial to view this content.
结果
有基于人口统计特征20组无显着差异。行为数据表明,目标探测任务是在困难的9-18岁之间的儿童和青少年适当的水平。在目前的研究中,控制系统正确识别目标(SD = 0.14)82.36%,而家族高危人群正确识别目标(SD = 0.17),76.8%。两个组识别情绪的图像时相比,中性图片精度降低(F(1,40)= 5.63,p值= 0.03)。
成像数据表明,实验条件导致显著激活...
Access restricted. Please log in or start a trial to view this content.
讨论
The modified emotional oddball paradigm in the current study has been shown to elicit differences in neural recruitment during selective attention and emotional processing in children and adolescents at risk for schizophrenia. While existing paradigms using the emotional oddball task have been used to investigate neural changes in adult populations with psychiatric illness8, the current paradigm may be particularly useful for measurement of vulnerability markers in younger age groups.
Access restricted. Please log in or start a trial to view this content.
披露声明
Dr. Perkins is currently receiving grant support from Janssen, and is a consultant to Dainippon. In the past, Dr. Perkins is or has been on speaker's bureau for Eli Lilly and AstraZeneca. Dr. Perkins has previously received grants from AstraZeneca, Bristol-Myers Squibb, Otsuka, Eli Lilly, Janssen and Pfizer; and consulting and educational fees from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Glaxo Smith Kline, Forest Labs, Pfizer and Shire. All other authors declare no biomedical financial interests or potential conflict of interest.
致谢
We thank Erin Douglas, Anna Evans, and Carolyn Bellion for their contributions to participant recruitment and clinical assessment. We also thank Michael Casp, Zoe Englander, Justin Woodlief, and James Carter for their contributions to data collection and analysis, and Robert M. Hamer for consultation on statistical analysis and editing of the manuscript. Finally, we thank the individuals and their families who participated in this study.
This study was supported by Conte center grant P50 MH064065 from the National Institute of Mental Health. Dr. Hart was supported by T32 HD040127 from the National Institute of Child Health and Human Development.
Access restricted. Please log in or start a trial to view this content.
材料
| Name | Company | Catalog Number | Comments |
| 3T MRI scanner | GE | BIAC 3T scanner (replaced) |
参考文献
- Kety, S. S., Rosenthal, D., Wender, P. H., Schulsinger, F. Mental illness in the biological and adoptive families of adpoted schizophrenics. Am J Psyc. 128, 302-306 (1971).
- Weinberger, D. R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychia. 44, 660-669 (1987).
- Nuechterlein, K. H., Dawson, M. E. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bul. 10, 160-203 (1984).
- Nuechterlein, K. H. The vulnerability/stress model of schizophrenic relapse: a longitudinal study. Acta Psychiatr Scand, Supp. 382, 58-64 Forthcoming.
- Keshavan, M. S. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosc. 3 (62), (2010).
- Kiehl, K. A., Liddle, P. F. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophr Re. 48, 159-171 (2001).
- Bramon, E. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimag. 27, 960-968 (2005).
- Dichter, G. S., Bellion, C., Casp, M., Belger, A. Impaired modulation of attention and emotion in schizophrenia. Schizophr Bul. 36, 595-606 (2010).
- Fichtenholtz, H. M. Emotion-attention network interactions during a visual oddball task. Brain Res Cogn Brain Re. 20, 67-80 (2004).
- Voyvodic, J. T. Real-time fMRI paradigm control, physiology, and behavior combined with near real-time statistical analysis. Neuroimag. 10, 91-106 (1999).
- International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings. Technical Report A-6. , The Center for Research in Psychophysiology, University of Florida. (2005).
- Smith, S. M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimag. 23, 208-219 (2004).
- Smith, S. M. Fast robust automated brain extraction. Hum Brain Map. 17, 143-155 (2002).
- Jenkinson, M., Bannister, P., Brady, M., Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimag. 17, 825-841 (2002).
- Jenkinson, M., Smith, S. A global optimisation method for robust affine registration of brain images. Med Image Ana. 5, 143-156 (2001).
- Woolrich, M. W., Ripley, B. D., Brady, M., Smith, S. M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimag. 14, 1370-1386 (2001).
- Beckmann, C. F., Jenkinson, M., Smith, S. M. General multilevel linear modeling for group analysis in FMRI. Neuroimag. 20, 1052-1063 (2003).
- Woolrich, M. W., Behrens, T. E., Beckmann, C. F., Jenkinson, M., Smith, S. M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimag. 21, 1732-1747 (2004).
- Genovese, C. R., Lazar, N. A., Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimag. 15, 870-878 (2002).
- Hart, S. J. Altered fronto-limbic activity in children and adolescents with familial high risk for schizophrenia. Psychiatry Re. 212, 19-27 (2013).
- Hariri, A. R., Bookheimer, S. Y., Mazziotta, J. C. Modulating emotional responses: effects of a neocortical network on the limbic system. Neurorepor. 11, 43-48 (2000).
- Gottesman, I. I., Gould, T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psyc. 160, 636-645 (2003).
- Glahn, D. C., Thompson, P. M., Blangero, J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Brain Map. 28, 488-501 (2007).
Access restricted. Please log in or start a trial to view this content.
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。