去除的分支和循环化合物的尿素络合法为 Uk'37 Paleothermometry
Overview
资料来源: 实验室的杰夫卡普-马萨诸塞大学阿默斯特
提到在上一视频中,有机溶剂萃取,产品总脂提取物 (TLE),经常是几百,如果不是,数千种不同化合物的复杂混合物。研究员是往往只对感兴趣的化合物少数。在我们两个的有机最高峰 (Uk'37和主战坦克/CBT),感兴趣的是只有 6 化合物 (2 脂肪酮和 4 isoprenoidal 甘油二烷基甘油 tetraethers)。在本系列中以前的两个视频中所述,可能为削减的分析样品中的化合物数量应用纯化技术。这些技术可能化学改变不需要的组件 (皂化),利用不同的复合化学结构 (柱色谱法),或使用不同的形状和尺寸的分子来包含或排除某些组件从分析 (尿素络合法)。不同的化学物质的原子结构导致一些有毒的有机化合物,形成长,狭窄,直链 (n-烷烃和脂肪酮),形成复杂的循环结构,别人形成高度支化结构,其他有机化合物和还有形成两个循环和分支结构 (GDGTs) (图 1)。不同形状和尺寸的试样中的化合物可以用于他们彼此分开,相同的方法如硬币清分机分隔不同教派 (尺寸) 的硬币。
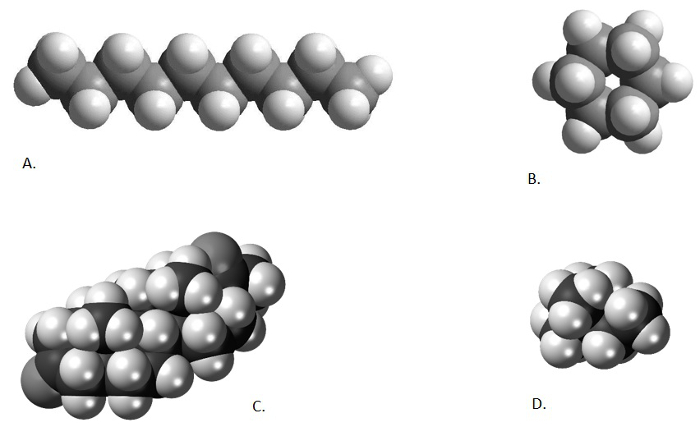
图 1。不同化学结构比较。正癸烷、 直链烷烃 (A; 从 http://www.bpc.edu/mathscience/chemistry),环己烷、 环烷烃 (B; 从 http://www.bpc.edu/mathscience/chemistry),一个类固醇、 聚环烃 (C; 从 www.wikiwand.com),和 2,2-二甲基丁烷异构烷烃 (D; www.wikimedia.com)。请点击这里查看此图的大版本。
Procedure
1.安装程序和材料的制备
- 获得总脂提取物 (TLE) 用溶剂萃取法 (超声、 索氏提取或加速溶剂萃取 (ASE))。
- 从任何化学的零售商购买以下材料: 燃烧高硼硅玻璃吸液管和灯泡;纯净水;正己烷;二氯甲烷 (DCM);甲醇;尿素。
- 试剂应该是纯洁的没有从碳氢化合物。另外,纯净水,可在实验室中用水净化系统。
- 获取与聚四氟乙烯衬里帽 4 毫升高硼硅玻璃小瓶。
2.方法
- 使混合了 2:1 DCM:Hexane (v: v)。
- 在锥形瓶,混合 200 毫升的 DCM 和 100 毫升的正己烷。
- 在甲醇 (100 毫克/毫升) 使尿素的混合物。
- 倒入锥形瓶 300 毫升的甲醇。
- 添加尿素 300 毫克。
- 搅拌自动搅拌盘,尿素完全溶解。
- 开始与干样品 4 毫升瓶中。
Results
Application and Summary
Tags
跳至...
此集合中的视频:

Now Playing
去除的分支和循环化合物的尿素络合法为 Uk'37 Paleothermometry
Earth Science
6.4K Views

确定空间方向的岩石层与布伦顿指南针
Earth Science
25.9K Views

利用地形图生成地形剖面
Earth Science
32.2K Views

制作一个地质剖面
Earth Science
47.3K Views

物理性能的一部分: 矿物晶体和劈裂
Earth Science
51.8K Views

物理性能的矿物二: 矿物分析
Earth Science
38.2K Views

火成岩火成岩
Earth Science
39.9K Views

火成岩侵入岩
Earth Science
32.4K Views

概述了 bGDGT 古气候学的生物标志物分析
Earth Science
5.4K Views

概述了 Paleothermometry 长链生物标志物分析
Earth Science
7.2K Views

超声提取沉积物的脂类生物标志物
Earth Science
9.9K Views

索氏提取脂类生物标志物的沉积物
Earth Science
18.6K Views

生物标志物提取沉积物-加速溶剂萃取
Earth Science
10.1K Views

转换的脂肪酸甲酯的皂化为 Uk'37 Paleothermometry
Earth Science
10.1K Views

总脂提取物与柱层析纯化
Earth Science
12.5K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。