15.2 : Acid/Base Strengths and Dissociation Constants
The relative strength of an acid or base is the extent to which it ionizes when dissolved in water. If the ionization reaction is essentially complete, the acid or base is termed strong; if relatively little ionization occurs, the acid or base is weak. There are many more weak acids and bases than strong ones. The most common strong acids and bases are listed below:
| Strong Acids | Strong Bases |
| HClO4 | LiOH |
| HCl | NaOH |
| HBr | KOH |
| HI | Ca(OH)2 |
| HNO3 | Sr(OH)2 |
| H2SO4 | Ba(OH)2 |
The relative strengths of acids may be quantified by measuring their equilibrium constants in aqueous solutions. In solutions of the same concentration, stronger acids ionize to a greater extent and so yield higher concentrations of hydronium ions than do weaker acids. The equilibrium constant for an acid is called the acid-ionization constant, Ka. For the reaction of an acid HA:

the acid ionization constant is written as
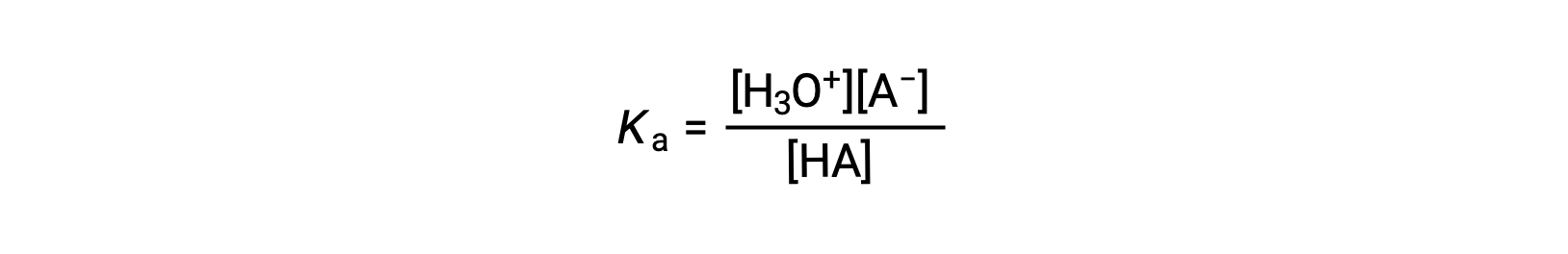
where the concentrations are those at equilibrium. Although water is a reactant in the reaction, it is the solvent as well, so we do not include [H2O] in the equation. The larger the Ka of an acid, the larger the concentration of H3O+ and A− relative to the concentration of the nonionized acid, HA, in an equilibrium mixture, and the stronger the acid. An acid is classified as “strong” when it undergoes complete ionization, in which case the concentration of HA is zero and the acid ionization constant is immeasurably large (Ka ≈ ∞). Acids that are partially ionized are called “weak,” and their acid ionization constants may be experimentally measured.
To illustrate this idea, three acid ionization equations and Ka values are shown below. The ionization constants increase from first to last of the listed equations, indicating the relative acid strength increases in the order CH3CO2H < HNO2 < HSO4−.
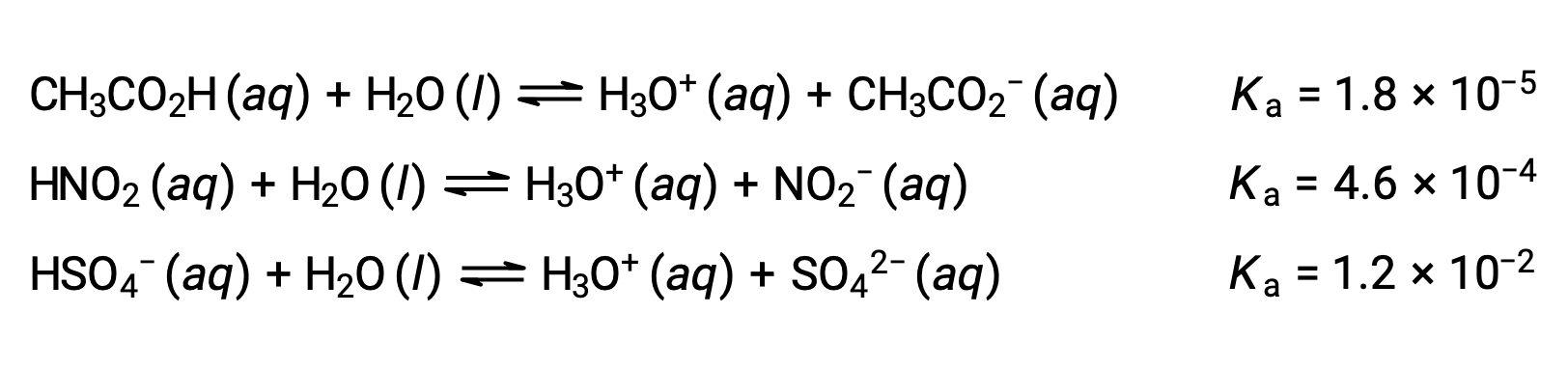
Another measure of the strength of an acid is its percent ionization. The percent ionization of a weak acid is defined in terms of the composition of an equilibrium mixture:
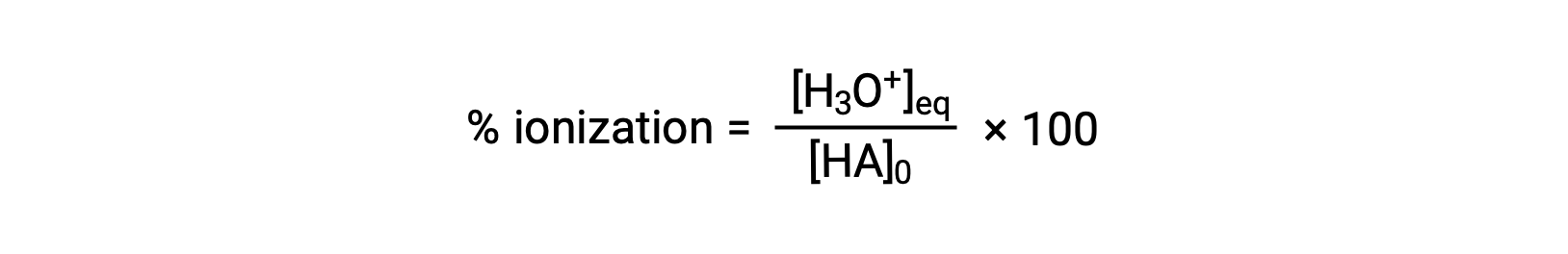
where the numerator is equivalent to the concentration of the acid's conjugate base (per stoichiometry, [A−] = [H3O+]). Unlike the Ka value, the percent ionization of a weak acid varies with the initial concentration of acid, typically decreasing as concentration increases.
Just as for acids, the relative strength of a base is reflected in the magnitude of its base-ionization constant (Kb) in aqueous solutions. In solutions of the same concentration, stronger bases ionize to a greater extent, and so yield higher hydroxide ion concentrations than do weaker bases. A stronger base has a larger ionization constant than does a weaker base. For the reaction of a base, B:
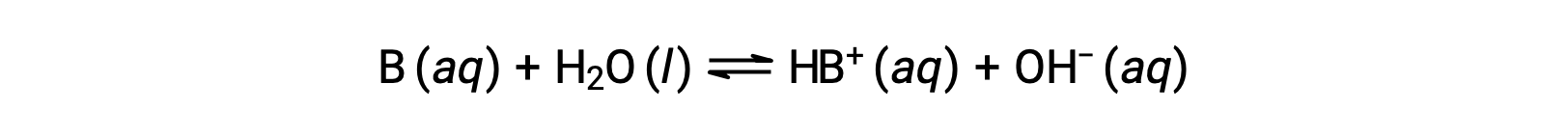
the ionization constant is written as
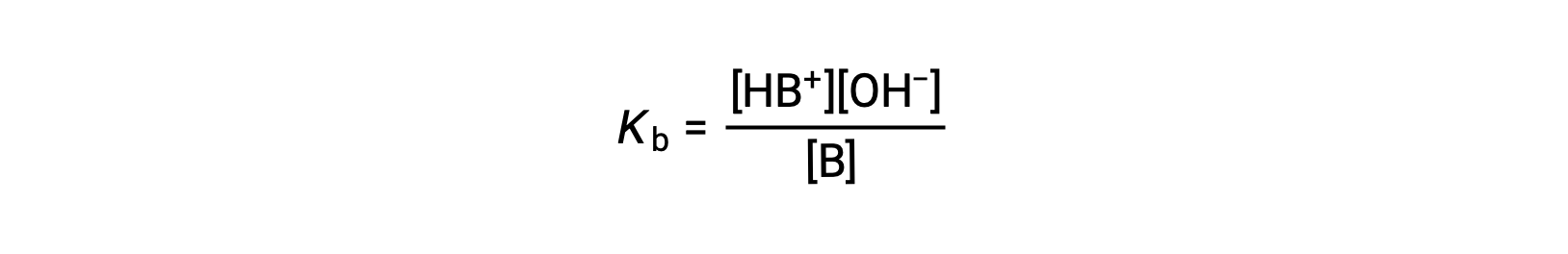
The inspection of the data for three weak bases presented below shows the base strength increases in the order NO2− < CH2 CO2− < NH3.
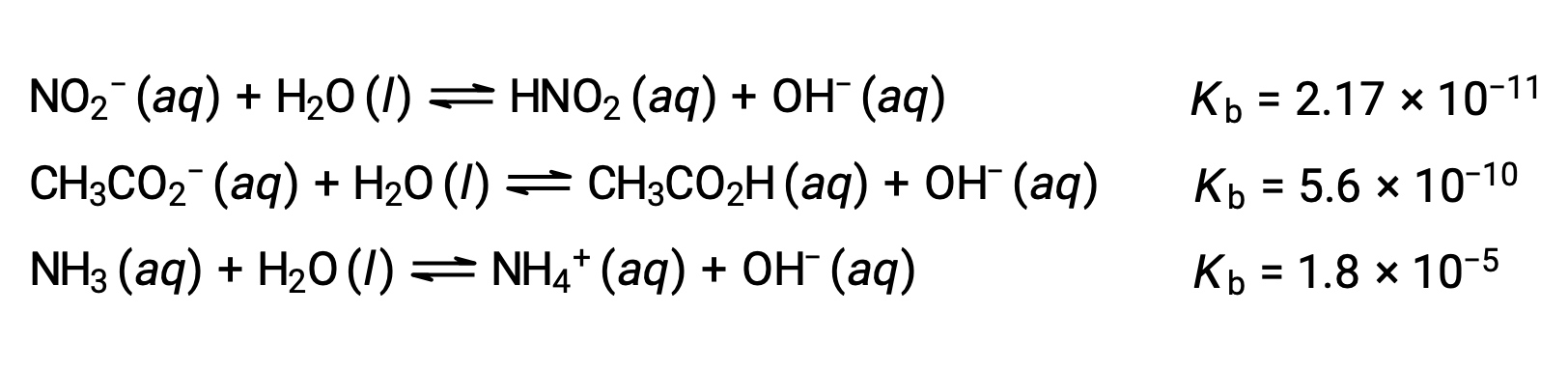
As for acids, the relative strength of a base is also reflected in its percent ionization, computed as
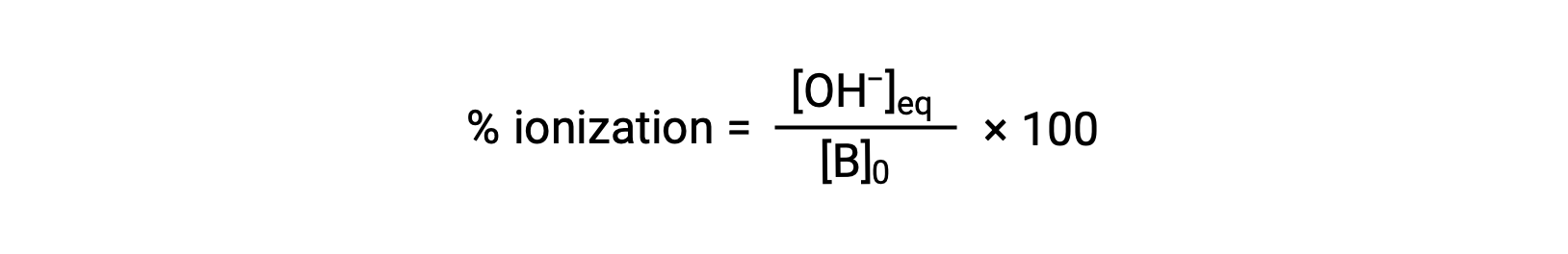
but will vary depending on the base ionization constant and the initial concentration of the solution.
This text is adapted from Openstax, Chemistry 2e, Section 14.3: Relative Strengths of Acids and Bases.
Aus Kapitel 15:

Now Playing
15.2 : Acid/Base Strengths and Dissociation Constants
Säuren und Basen
60.1K Ansichten

15.1 : Bronsted-Lowry Säuren und Basen
Säuren und Basen
90.8K Ansichten

15.3 : Wasser: A Bronsted-Lowry-Säure und Base
Säuren und Basen
49.9K Ansichten

15.4 : pH-Skala
Säuren und Basen
68.3K Ansichten

15.5 : Relative Stärken von konjugierten Säure-Base-Paaren
Säuren und Basen
45.3K Ansichten

15.6 : Starke Säure- und Basenlösungen
Säuren und Basen
31.4K Ansichten

15.7 : Schwach saure Lösungen
Säuren und Basen
37.6K Ansichten

15.8 : Schwache Basislösungen
Säuren und Basen
22.5K Ansichten

15.9 : Mischungen von Säuren
Säuren und Basen
19.5K Ansichten

15.10 : Ionen als Säuren und Basen
Säuren und Basen
23.5K Ansichten

15.11 : Bestimmung des pH-Werts von Salzlösungen
Säuren und Basen
43.3K Ansichten

15.12 : Polyprotische Säuren
Säuren und Basen
29.0K Ansichten

15.13 : Säurestärke und Molekularstruktur
Säuren und Basen
30.7K Ansichten

15.14 : Lewis-Säuren und -Basen
Säuren und Basen
43.4K Ansichten
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten