Method Article
Constructing an Olfactometer for Rodent Olfactory Behavior Studies
In This Article
Summary
This protocol describes the construction of an olfactometer for go/no-go olfactory behavior experiments. Step-by-step instructions, along with images, are provided to ensure the successful construction of the olfactometer. Information for troubleshooting issues encountered during the process is also included.
Abstract
The use of olfactometers to study rodent behavior and brain activity during olfactory tasks is crucial for understanding brain circuits. These sophisticated devices allow researchers to precisely control and deliver odor stimuli, enabling the investigation of complex olfactory processes in rodents. Although commercially available olfactometers are convenient, they present challenges when technical issues arise, often requiring costly assistance and potentially disrupting research timelines. This article details the construction of a custom olfactometer specifically designed for mouse olfactory behavior experiments, providing a comprehensive list of parts and step-by-step instructions. The olfactometer is controlled through MATLAB, offering a user-friendly interface for researchers. Importantly, the open-source code allows users to modify and adapt the system, tailoring behavioral tasks to meet specific experimental needs. Building a customized olfactometer empowers users with the knowledge and capability to perform custom experimental design and troubleshooting independently, saving both time and resources. This approach not only enhances research flexibility but also fosters a deeper understanding of the equipment's functionality, ultimately leading to more robust and reliable olfactory studies in rodents.
Introduction
The intricate mechanisms underlying olfactory decision-making offer fascinating insights into the remarkable complexity of the brain's sensory processing system1,2,3. Within the olfactory bulb of mice, a vast array of olfactory sensory neurons converge on approximately 2,200 glomeruli, each innervated by neurons expressing the same olfactory receptor4. Remarkably, even single synthetic odorants can stimulate a substantial portion of the roughly 1,100 olfactory receptors in mice5,6. However, the challenge extends beyond initial odorant detection. The temporal dynamics of odorant arrival, influenced by the rhythmic act of sniffing, further enrich the sensory landscape, adding layers of information for the brain to decipher. Compounded by the complexity of natural stimuli, such as conspecific urine, which contains hundreds of odorants, the olfactory system faces the formidable task of disentangling intricate patterns of glomerular activation to differentiate between various scents7,8.
To address this challenge, the brain orchestrates neural activity across multiple regions, including the piriform cortex, lateral entorhinal cortex, hippocampus, olfactory tubercle, prefrontal cortex, and even the cerebellum9,10,11,12,13,14. Within these circuits, pyramidal cells in the piriform cortex integrate and modulate information relayed by mitral cells, while other brain areas contribute unique roles in shaping olfactory perception15,16,17. Moreover, the brain's processing of olfactory stimuli is dynamically influenced by contextual factors, underscoring the adaptability and sophistication of the olfactory decision-making process.
This article describes the construction of a custom olfactometer that enables computer-controlled assessment of the behavioral performance of freely moving mice engaged in a go/no-go task. The animal is water-restricted for 2 days, and subsequently, the amount of water is limited to 1.5-2 mL per day. To ensure that the mouse is not dehydrated, ensure that the animal’s weight does not fall below 85% of the weight measured before training. When properly controlled for dehydration, the procedure is not detrimental to the mouse. In this associative learning task, the water-deprived mouse initiates a trial by licking a water delivery spout located within an odorant delivery nose cone. One of two odorants is delivered 1-1.5 s after the animal initiates the trial. If the odorant is the rewarded (S+) odorant, the mouse receives a water reward if it licks at least once in each of four 0.5-s time windows (a Hit). Otherwise, the mouse receives no reward (Miss). If the animal receives the unrewarded odorant (S-), no reward is delivered, and if the mouse licks in each of the four time windows (False Alarm, FA), a time delay is imposed before the start of the next trial. If the animal fails to lick in one of the time windows, the trial is counted as a correct rejection (CR), and no time delay is applied. The percentage of correct performance is calculated as the percentage of trials in which the mouse scores a Hit or CR in a twenty-trial window:
Percent correct = 100 ((Hit + CR) / 20)
There are two key issues to ensure the proper functioning of olfactometers designed to assess go/no-go olfactory behavior. First, the olfactometer must monitor the mouse's responses in real time to deliver odorant and water rewards accordingly. This olfactometer is achieved by monitoring licks either by measuring the resistance between the waterspout and the chamber floor or by sensing capacitance18. A MATLAB program then uses this information to make decisions on odorant delivery and water reward. The second issue is the need for reliable, reproducible odorant delivery. This olfactometer is achieved by actuating valves that equilibrate odorant-saturated air with carrier air, which is then delivered to a nose cone. Air is equilibrated with the odorant by bubbling it through a mineral oil-diluted odorant solution. The concentration of the odorant is measured with a photoionization detector and can be calculated based on vapor pressure and activity coefficient, following procedures described by Williams and Dewan18,19.
Protocol
All experiments were conducted according to protocols approved by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee. The animals used in this study were male CaMKIIα WT mice, aged two months at the time of tetrode implantation. Tetrode implantation was performed as described in Villanueva et al. Wait for at least a week after the surgery before starting water deprivation. Details of the reagents and equipment used in this study are provided in the Table of Materials.
1. Board and soldering of single-pole, single-throw (SPST) momentary push buttons
- Obtain the custom-made white baseboard with holes to fasten the racks for odor valves, flow meters, SSR48 board, and other components shown in Figure 1A.
NOTE: The white baseboard used in this study was produced by the Machine Shop of the Neurotechnology Center at the CU Anschutz Medical Campus (Figure 1A). The design files are available at: https://a360.co/3Ag2QvF - Add 1-inch screws and 3/4 inch spacers for placement of the SSR48-RACK. The screws are located on the top right of the back of the whiteboard (Figure 1A).
- Mount the SSR48-RACK. The SSR48-RACK will be located on the top left side of the back of the whiteboard (Figure 1C).
- Make holes (0.4 cm) to place the screw terminal strip blocks on the back of the whiteboard. The screw terminal strip blocks will be located in the middle right of the back of the whiteboard (Figure 1D).
- From top to bottom: The first 4 screw terminal strip block will be used to connect wires for 24 V. The next screw terminal strip block will be used for 5 V wires. Leave one screw terminal strip block empty, and the last 4 screw terminal strip block will be used to connect ground wires.
- Drill holes (0.8 cm) into the control box for placement of the SPST momentary push button switches. The control box will be located at the front bottom of the whiteboard (Figure 1E).
- Set up the SPST momentary push button switches. Solder two wires to the SPST momentary push button switch. It is preferential to use two different colors, such as red and black or green (Figure 1F).
- Attach the SPST momentary push button switch to the control box. The push buttons come with a nut that is used to attach to the control black box (Figure 1G).
- Secure the wires by twisting or securing them with tape to keep them together and organized.
NOTE: The 24 V power is used to power the pinch valves and odor valves, and 5 V power is used for the lick circuit. - Place the odor valves into the slots of the odor valve rack located in the center of the whiteboard (Figure 2A).
- Peel the wires that come off the valves and solder one wire from each valve onto a thicker wire. Place one wire into the ground on the screw terminal strip blocks on the back of the whiteboard and a second wire into the corresponding pin in the SSR48-RACK. For example, odor valve 1 goes to pin 1, odor valve 2 goes to pin 2, etc (Figure 2B).
- Connect pins 1 to 8 in the SSR48-RACK to two pinch valves each (in and out valves for the mineral oil equilibration vials). For each valve, connect one wire from a push button to the 24 V power and the other wire to the pin in the SSR48-RACK that is connected to the valve. The other wire goes from the valve to the ground. Refer to Figure 2B,C for placing the wires into their place.
- Place the water valve and final valve in the corresponding slots in the valve plate. The slot is located in the middle of the whiteboard. See the valve plate in Figure 1 and the placement of the valves in Figure 3A.
- Connect the water valve and final valve to ground and pins 17 and 18, respectively, in the SSR48-RACK. Connect push buttons to 24 V and to pins 17 and 18 (Figure 2B).
2. Power supply
- Buy a power supply and an extension cord. Information regarding the specific type of power supply can be found in the Table of Materials. The power supply will provide 24 V (V3) and 5 V (V1) outputs and ground to the olfactometer.
- Cut off the plug of the power cord for the power supply. Specifically, the female part of the extension cord. Once it is cut, three wires can be seen. The green wire is connected to G (ground) on the power supply, and the other two wires (white and black) are connected to L and N, respectively, of the 120 V AC input to the power supply (Figure 3B).
CAUTION: The 120 V power wires are exposed, and there is a risk of injury by electric shock. It is best to cover it with an insulator. - Cut one end of the wire powering the SSR48-RACK. Connect one of the wires to the G screw in the power supply and the second to the V1 of the power supply (Figure 3C).
- On the power supply, connect one wire from G2 to the ground on the screw terminal strip blocks (Figure 2C).
- On the power supply, connect one wire from V1 to the 5 V screw terminal strip blocks (Figure 2C).
- On the power supply, connect one wire from V3 to the 24 V screw terminal strip blocks (Figure 2C).
3. Lick Sensor board
- Obtain a 400-tie points breadboard and run a wire that connects from tie points B7 to B15 (Figure 3D).
- Connect one end of a wire to tie point 6+ and the other end to tie point C22 of the breadboard (Figure 3D).
- Connect one end at D16 and the second end to G22 of the breadboard (Figure 3D).
- Connect one end of a wire to slot I22 and the second end to 29- of the breadboard (Figure 3D).
- Connect one end of a wire to slot 20A and the second end to 29A of the breadboard (Figure 3D).
- Connect one end of a wire to 21B and the second end to 28B of the breadboard (Figure 3D).
- Connect one end of a wire to slot 1+ of the breadboard and the second end to the 5 V of the screw terminal strip blocks (Figure 3D).
- Connect one end of a wire to slot 1- of the breadboard and the second end to the ground of the screw terminal strip blocks (Figure 3D).
- Connect one end of a wire to slot C7 of the breadboard and the second end to pin 27 on the SSR-48RACK (Figure 3D).
- Connect one end of a wire to slot 28C of the breadboard, and the second end will have an alligator clip attached that will connect to the metal part of the waterspout (Figure 3D).
- Connect one end of a wire to slot 20B of the breadboard and the other end to the middle terminal of the potentiometer (Figure 3D). The two terminals connected to the resistance elements of the potentiometer are connected to ground and 5 V. The middle terminal is connected to the breadboard.
- Obtain 21 mΩ resistors. For the first one, connect one end to 19A and the second end to 20D. For the second one, connect one end to 22C and the other end to 21D. Connect to the potentiometer (Figure 3D).
- Obtain one kΩ resistor. Connect one end to 14C and the second end to 19C. Connect to the potentiometer (Figure 3D).
- Obtain one 120 Ω resistor. Connect one end to 7D and the second end to 7H (Figure 3D).
- Obtain an LED light. The color does not matter. Connect one LED wire to 7J and the second wire to 6- (Figure 3D).
- Obtain two operational amplifiers (op-amps). The connections for the first one are E10 to E16, F10-F16 (Figure 3D).
4. Air and water supply
- Place two flowmeters (a 2 L/min and a 50 cc/min) into the flow meter holders. Figure 4A shows the overall airflow system, and Figure 4B shows the magnified view of the flow meters.
- Obtain an aquarium pump to provide 2 L/min airflow. The aquarium pump model used here has two outputs (see Table of Materials). Connect a small piece of tubing from each of the two outputs of the aquarium pump to the two inputs of a T connector (Figure 4B).
- Connect a piece of tubing from the output of the T connector to the input of an active carbon filter (Figure 4B).
- Connect tubing from the output of the carbon filter to a T connector and connect the two outputs of the connector to a ball valve to adjust the airflow rate (Figure 4C).
- Connect the output of each ball valve to the input of the flow meters (Figure 4D).
- Connect the output of the 50 cm/min flow meter to the top manifold, providing air to the 40 mL odor equilibration vials with odorants diluted in mineral oil (Figure 4E).
- Connect the output from each odor vial to the corresponding input in the lower manifold.
- The tubes connecting the odor vials to the manifolds are pinch valve tubes that are opened by the two separate pinch valves. Place the tubing into the pinch valves.
- Connect the output of the 2 L/min flow meter to the input of the side input of the lower manifold.
- Connect the output of the lower manifold to the input of the final (diverting) valve (Figure 4F).
- Connect the default on output of the final valve to the odor delivery tube in the go/no-gochamber. Connect the default off output of the final valve to an exhaust tube (Figure 4G). This results in continuous non-odorized airflow of 2 L/min when the final valve is off.
- For each trial, ensure the final valve turns on when the animal licks, taking the air to the exhaust, and simultaneously the odor valve turns on. This results in the equilibration of odor in the background airflow.
- After 1-1.5 s, ensure that the final valve turns off, diverting the air back into the chamber. This results in a sharp increase in odorant concentration. After 2.5 s, the odor valve turns off, and the odor concentration returns to 0.
- Connect an 18 G needle to the tip of the 5 mL syringe that will be used to deliver the water reward (Figure 4H).
- Connect one tube (2 mm diameter) to the tip of the needle (Figure 4H).
- Connect the other end of the tube to the input of the water valve. One might need to cut a tube of a different diameter to fit the input of the water valve (Figure 4I).
- Connect the tube from the output of the water valve to the lickspout (Figure 5A).
5. Connecting the olfactometer to the computer and installing the software
- Connect the SSR48-RACK to the DIO96H/50 with a 100-pin female-to-female connector. Connect the USB cable from the DIO96/H50 to the computer (Figure 5B).
- Download the latest version of mccdaq software and drivers and InstaCal.
NOTE: InstaCal is the program that tests the communication between the computer and DIO96/H50. Download the latest software and drivers here: https://www.mccdaq.com/software-downloads.aspx. - Run InstaCal. Make sure the "Universal Serial Bus" lists the Board # as the correct number, usually #1 = Board #1 USB-DIO96H/50.
- Download MATLAB.
- Download the MATLAB programs to run the olfactometer from https://github.com/restrepd/dropc.
- Open MATLAB as an Administrator and set the path so that MATLAB recognizes the programs. On the Home tab in the MATLAB environment, click on Set Path in the Environment section. This opens a dialog where you can add folders in the search path.
- Run daqregister('mcc'). Change the board number in dropcInitializePortsNow.m.
NOTE: handles.dio = digitalio('mcc',1); %(1 or 0 depending on the computer). - Test dropcspm.m by performing a dry run where the user "responds" to each trial by connecting the electrical loop between the lick spout and the metal floor of the chamber that is grounded.
NOTE: The olfactometer is now ready to use. Information on how to train the mouse is found in Nicole Arevalo et al.20.
6. Animal experiments
- Prepare the animal subjects carefully to begin the experimental process. Weigh each mouse individually using a calibrated scale and record the weight in a laboratory log. Monitor this crucial data throughout the study to track the animals' health and address any weight changes promptly.
- Place the mice gently into the specially designed mouse chamber after weighing. Activate the sensors and stimuli delivery systems for the olfactory discrimination task. Ensure the chamber minimizes stress on the animals while maintaining precise control of experimental conditions. Clean the chamber with unscented soap and water between sessions. Do not use alcohol, as the plexiglass will become brittle.
- Ensure that the animal is comfortable in the chamber. Initiate the MATLAB program to control experimental parameters such as delivering odor stimuli (2.5 s), dispensing water, and recording responses. Analyze data in real time to obtain immediate feedback on the animal's performance.
- Monitor and analyze the animal's performance continuously. Look at the calculated proficiency score based on the percentage of correct responses. Aim for each animal to achieve a performance score of 80 or greater, which marks the threshold for task proficiency.
- Begin the new phase of the experiment once the animal consistently achieves a proficiency score of 80 or higher, indicating mastery of the initial odor pair discrimination. Reverse the odor pair, leaving the previously rewarded scent unrewarded and vice versa.
- Test the animal's cognitive flexibility and ability to unlearn and relearn associations, thereby gaining valuable insights into the plasticity of olfactory learning in mice.
Results
Following the protocol described here, an olfactometer can be set up to test the go/no-go behavior of mice differentiating between odors. Figure 6A shows the behavior of a mouse during the first day of training in the go/no-go task, using ethyl acetate as the S+ odorant and a combination of ethyl acetate and propyl acetate as the S-. The percent correct is calculated as the percentage of trials in which the mouse scores a hit or correct rejection. Initially, the mouse started at 50% correct because it licked in response to both odorants. However, after several trials, it learned to lick only for the S+ and stopped licking for the S-. Figure 6B shows the percent correct for the last day of the go/no-go task in the forward direction, where the animal achieved proficiency with performance at 80% or higher. At this point, the odorants were reversed (REV), with ethyl acetate as the S- odorant and the combination of ethyl acetate and propyl acetate as the S+. Figure 6C shows the percent correct on the first day of the go/no-go task in the reverse direction, where the mouse's performance dropped to 10%. F shows the mouse's performance on the last day of reversal, where it again achieved proficiency.
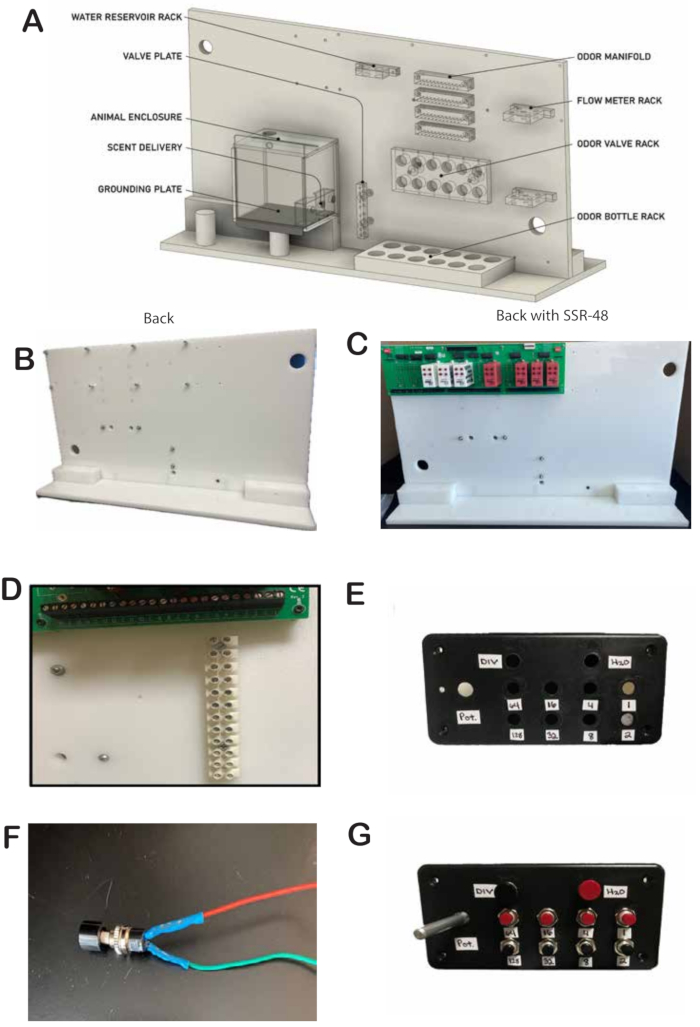
Figure 1: Whiteboard front side of the olfactometer and wiring. (A) The olfactometer dimensions are 22" W x 16" H x 8.5" D, shown without wiring or interface/lick boards, as delivered from the machine workshop. Holes are pre-drilled for odor valves, thermometers, water and final valves, water syringe, odor bottle rack, odor valve rack, and the mouse chamber. (B) The olfactometer prepped with the 8 bolts required to mount the SSR48-RACK on the back left side. (C) Olfactometer with SSR48-RACK mounted, including attached relays. (D) Screw terminal strip blocks added to the olfactometer, with designated sections for 12 V, 5 V, and ground. (E) Control black box with drilled holes for SPST momentary push button switches, with tape labeling each button by valve control. (F) SPST momentary push button switch with two color-coded, shrink-tubed wires soldered to protect exposed areas. (G) SPST momentary push button switch mounted to the control black box and secured with the included hex nut. Please click here to view a larger version of this figure.
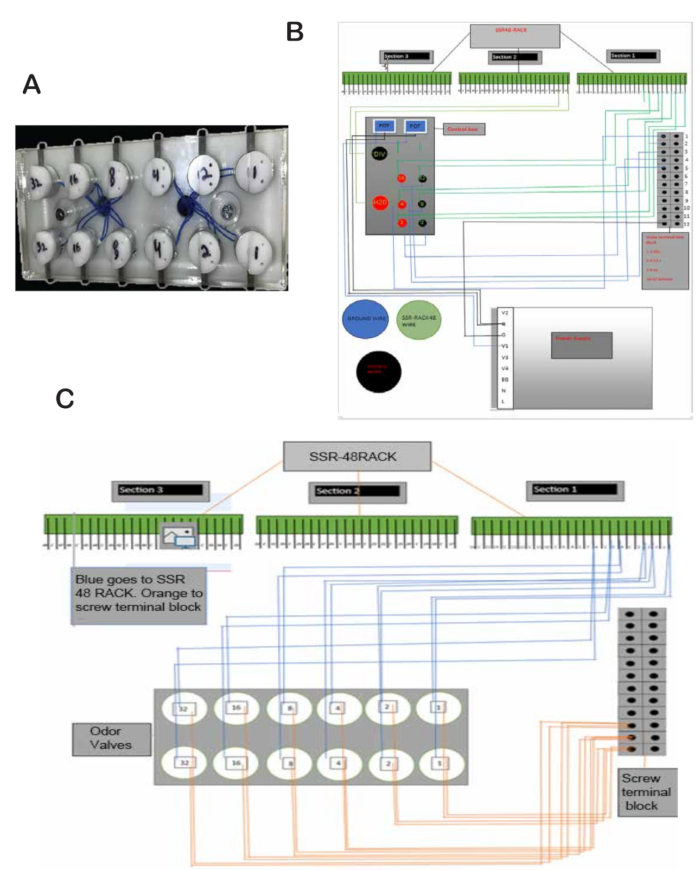
Figure 2: Odor valves and schematics. (A) Odor valves fitted securely in slots and fixed with screws. (B) Schematic of odor valve wiring to the SSR48-RACK and screw terminal strip blocks. (C) Wiring schematic of the control black box, power supply, SSR48-RACK, and screw terminal strip blocks. Please click here to view a larger version of this figure.
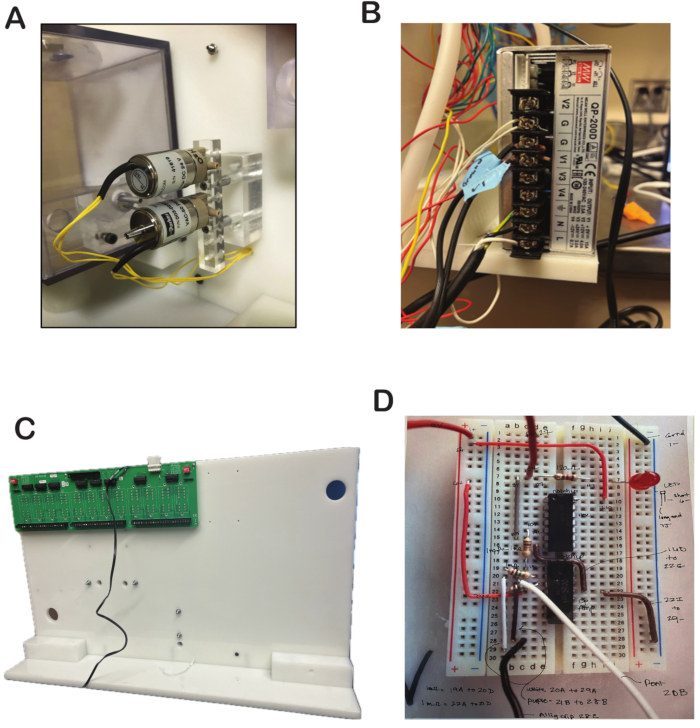
Figure 3: Water and final valve setup with power supply. (A) Water and final valves added to the designated slots in the olfactometer and secured with screws. (B) Power supply wiring connected to power the olfactometer. (C) Power wiring for the SSR48-RACK. (D) Lick sensor with connected components, including resistors, wires, LED, and operational amplifier. Please click here to view a larger version of this figure.

Figure 4: Air supply system with flow meters and tubing. (A) Flow meters attached to the rack with screws. (B) Aquarium pump connected to tubing, joined with a T-joint. (C) A carbon filter with tubing attached at the output, with connections to individual regulators. (D) Tubing from regulators connected to flow meter inputs. (E) Tubing attached to flow meter outputs. (F) Tubing from the manifold to the final valve input. (G) Final valve with tubing connected to the olfactometer odor port. (H) 5 mL syringe filled with water, tubing attached to an 18 G needle. (I) Tubing connected to the water valve input. Please click here to view a larger version of this figure.
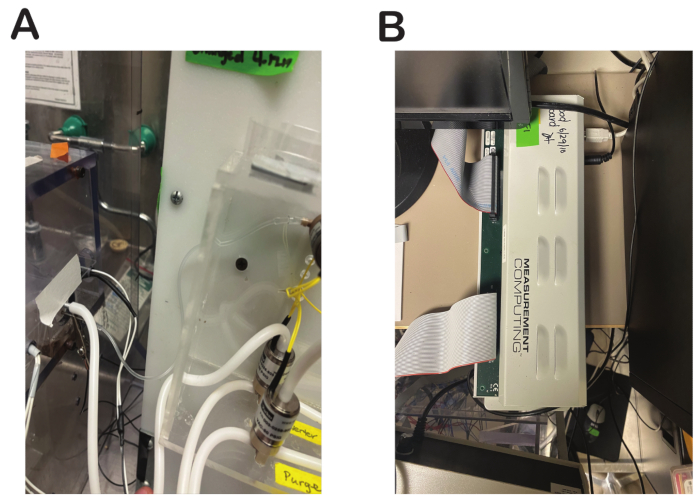
Figure 5: Final water valve connections and system overview. (A) Tubing from water valve output to the lixit in the olfactometer. (B) Connection of the olfactometer to the DIO96H/50 using a female-to-female cable. Please click here to view a larger version of this figure.
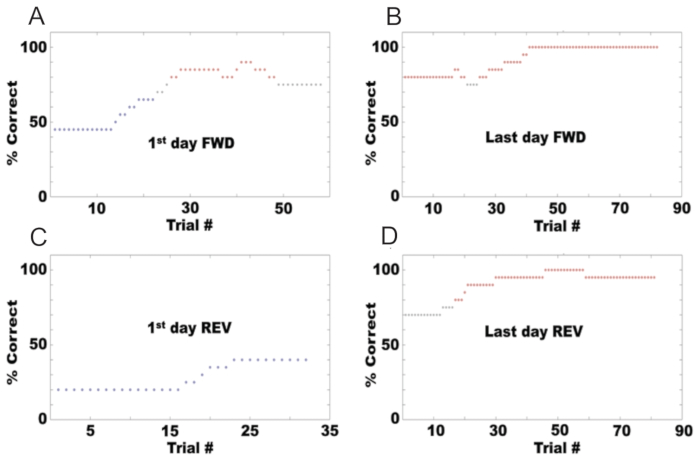
Figure 6: Behavioral performance example in a go/no go task for a mouse. Percent correct responses in each session are displayed for: (A) The first day of forward conditioning (S+: 1% isoamyl acetate, S-: mineral oil). (B) The last day of forward conditioning. (C) The first day after reversal (S+: mineral oil, S-: 1% isoamyl acetate). (D) The last day of reversed conditioning. Please click here to view a larger version of this figure.
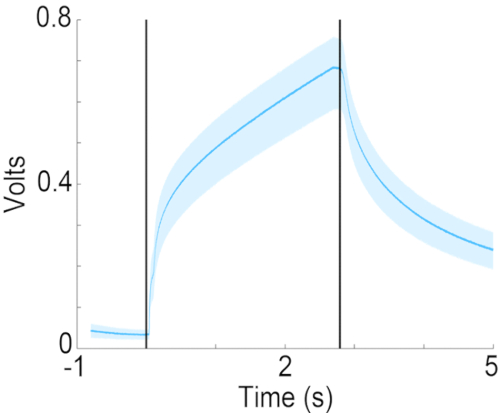
Figure 7: Concentration time course of isoamyl acetate in the odor port. Measured concentration of 10% isoamyl acetate (diluted in mineral oil) in the odor port, using a photoionization device (PID). Vertical lines indicate the start and end of odor delivery. Please click here to view a larger version of this figure.
Discussion
A comprehensive step-by-step guide for constructing an olfactometer designed for odor association tasks can be found in the literature. Researchers may encounter various challenges during the assembly and operation of the device, but fortunately, there are established troubleshooting methods to address these issues. Once properly constructed and calibrated, the olfactometer serves as an invaluable tool for scientists conducting experiments related to olfaction, enabling precise control and delivery of odor stimuli.
Critical steps
The MATLAB version downloaded should be 2015, as the code written is compatible with this version, and using any other version may lead to issues. It is important to verify that the correct board is selected in instacall. The installation of mcc.dill can be accomplished by executing daqregister('mcc') while logged in as a MATLAB administrator.
Troubleshooting
At the beginning of each training week, run the calibration software (InstaCal) to make certain that the PC and the olfactometer are interfacing correctly. Open this program, click on the board, and click on Digital Calibration. The airflow rate needs to be checked. The background airflow should be 2 L/min, and the flow to the odor equilibration vials should be 50 mL/min. It is extremely important to routinely check for airflow rate at the output of the olfactometer.
Before placing a mouse, it is important to ensure the following parameters are tested: (1) Odor valves: The odor valves should be clicked when pressing the button on the black box. Air tubing should bubble into the mineral oil, with no mineral oil trapped in the tubing. (2) Odor tubing: If odors do not produce bubbles, the tubing may be blocked where it typically pinches near the valve. Tubing replacement may be necessary. (3) Final valve and water valve: The final valve should open correctly, with the tubing checked for proper function. For the water valve, any air blocking water flow should be cleared, allowing water to flow into the lixit. (4) Airflow: The flow meters need to appear calibrated and positioned appropriately. (5) Relay lights: Ensure that the "lights" on the relays activate correctly during the experiment. (6) Water reward relay: When a water reward is dispensed, the relay above the water should blink as it dispenses the reward. (7) Odor and water reward relay: When an odor is paired with a water reward, the red relay above the corresponding valve number should blink red during the reward.
Limitations
The go/no-go task tests the ability of the mouse to test 2 odors. In order to run the task, the animal must be run through several sessions. This is not a high throughput technique for testing odor discrimination. The olfactometer is designed to test olfactory stimuli. It is not a multisensory testing apparatus. However, modifications can be made to test other sensory inputs.
This article describes a liquid dilution olfactometer where air bubbling through the odorant diluted in mineral oil at a rate of 50 mL/min is pre-equilibrated with the background airflow at 2 L/min. For this odorant delivery design, the kinetics of odor dilution in the carrier airflow determines the speed of the increase in the concentration of the odor in the background airflow. As shown in Figure 7, while the concentration increases within 200 ms to half of the final concentration, the rate of concentration change slows down beyond half a second. While this configuration does not produce a square step change in odor concentration, it has been used successfully to study odor discrimination and detection21. If the experimental protocol requires a step change in odor concentration, the design for odorant delivery should be modified to three continuous 2 L/min background airflow channels where odorants are continuously delivered into the background airflow of two of the background airflow channels. The third delivery channel would deliver air equilibrated with mineral oil. In this case, diverting valves would be used to divert one of the two odorants or odor-free air into the odor port. This would result in step increases in odorant concentration at the odor port (also previous reports19,22). Regardless, it is key to document the time course for the change in odor concentration using a photoionization detector.
The olfactometer described here is designed for mouse behavioral experiments, however this design has been used in the past for rats. The main difference is that it is necessary to increase the size of the chamber for studies with rats23. Finally, this olfactometer assesses the olfactory behavior of a single mouse. A high throughput automated olfactometer has been described to test multiple mice24.
Significance
This protocol describes a custom-made olfactometer, which reduces costs compared to other available methods.
Future applications
The olfactometer is designed specifically for use with mice and requires modification for use with other animals, such as rats. Additional features, such as a multi-electrode recording system (e.g., a multi-electrode recording board), an Arduino Uno board, or a camera, can also be incorporated.
Disclosures
The authors have nothing to disclose, and there are no competing financial interests.
Acknowledgements
This research was supported by grants from NIH grants K01 NS127850-01, R25 NS080685, R01 NS081248, and DC000566. We would like to thank all members of the Restrepo's and Ramirez-Gordillo's lab for their support.
Materials
| Name | Company | Catalog Number | Comments |
| 2 1/8’’ modular ic breadboard socket | found on: amazon.com | ASIN : B004MCSOQY | Powers the lights and lick censor |
| 500 piece assorted carbon film resistors 1/4 watt | Found on amazon.com Brand:bojack | Resisters will go on the BreadBoard socket | |
| 50k-ohm linear taper potentiometer | Brand:TWTADE Found on: Amazon.com | Allows to the components of the olfactometer Power supply: Ac 220v-6A Shift diameter: 6mm/0.2” Shaft length: 15mm/0.59” mounting thread. Knob size 15/17mm/0.6 x 0.67”(d*H) the adjustment of power | |
| 5mm red LED | Found on: Amazon.com Brand:EDGELEC | Pre-wired with built in Resistor; 5mm round top bulb and wired LED's-Easy Connection with 3-6V DC Drive it, 7.9 inch long wires. Wattage: 1 Watts | |
| 6 position dual row brrier strip | Found on: Digikey.com | Base Product Number 1546306 | Used for power and ground depending on how it is connected Voltage rating:300v Current rating (amps):20A Wire gauge:12-22 AWG |
| 96 high current 50 pin connector female to female | Found on: Amazon.com Brand: IIVVERR | Part Number:f5c953ee65a980d | Weight: 109G Pitch:2.54mm Total size: 50x6.4cm/2x2.5 inch |
| Aquarium pump -AAPA7.8L 125 GPH, 2 OUTLETS 3W | Found on:Amazon.com Brand: Hydrofarm store | This will connect to the double open end cold water housing and will be used to power the air on the machine | |
| Barbed Tee connector polyethylene 1/4” | Found on: Uplastic.com | Item number:62200 | Connects different air hoses to each other |
| Barbed Tee connector polyethylene 3/16” | Found on: Uplastic.com | Item number:62063 | Connects different air hoses to each other |
| BD general use precision glide hypodermic needle 18 G ½ | Found on: Medneedles.com | Item number: BD 305195 | Used for the water system |
| Black box/manual control box | Brand:Otdorpatio Found on: Amazon | n/a | Used as the control box Dimensions: 3.94x2.68x1.97 |
| Cable, pc power supply internal connections 10ft | Found on: Amazon.com | Connects to the power supply 40 watts | |
| Cflex tubing, white ¼” id x 3/8” OD | Found on:uplastic.com | Item number:54033 | Tubing used in the air system |
| custom-made white base board with holes to fasten the racks for odor valves, flow meters, SSR48 board | The Machine Shop of the Neurotechnology Center in the CU Anschutz Medical Campus | ||
| Diverter valve—miniature inert liquid valve, 3 way | Found on:Radwell.com | Part number:003-0258-900 | 1/8th BARB 24VDC UP TO 1500 SCCM 4.2W |
| Double open end cold water housing with blue sump | Found on:GRAINGER.COM BrandPENTAIR/PENTEK | Compatible Mfr. Model Number150295; 150578; 151117; 151118; 151120; 155003; 244043; 244686; 244687 | |
| Fisherbrand glass EPA vials | Found on: fishersci.com | Catalog no. 02-912-379 | Used for odors |
| Fitting reducer 1/4” x 1/8” | Found on: uplastic.com | Item number: 64370 | Adapter used in the air system hoses. |
| Hard Tubing, intramedic polyethylene, 0.045 (ID) 1 x 100 | |||
| High infrared LED | Found on amazon.com Brand: gikfun | Memory clock speed 1mhz | |
| Ic opamp gp 4 circuit 14dip | Found on: Amazon.com Brand:BOJACK | Operating Voltage 50 Volts Maximum Voltage 50 Volts | |
| Jumper wire kit | Found on: Amazon.com Brand:Elegoo | Item Dimensions LxWxH: 0.04 x 8.27 x 0.04 inches | |
| Mini spst momentary switch | Brand:Radioshack Found on: Amazon.com | B000TLWZM6 | Used for the odor valves Operating volage: 250V Current rating:1 Amps |
| Multimeter | Found on Amazon.com Brand:AstroAl | Accurately measures AC/DC Current, AC/DC Voltage, Capacitance, Frequency, Duty Cycle, Resistance, Diode, Continuity and Temperature | |
| Needle nose | Found on:amazon.com Brand:WorkPro | Will be used to bend wires | |
| Odor valves | Found on:Radwell.com | SKU: 192833415 Part number:225T031 | Pumps odors from odor vials. 30 PSIG 12 VDC |
| Phototransistor | Found on: Amazon.com Brand:HILETGO | Voltage: 1.3-1.5V Receive Range: (NM) 400-1000 Head Size: 5mm x 5mm / 0.2" x 0.2"(D*L) | |
| Phototransistor and LED as pair | |||
| Pipe adapter 3/4” x 1/4” | Found on: uplastic.com | Item number: 64807 | Adapter used in the air system hoses. |
| Pipe adapter for water housing pump- m ¾ x1/4” | Usplastic.com | Item number: 64807 | Temperature range -50f to 275F Maximum pressure: 150psi Weoght 0.0015 lbs |
| Power supply 12v 30A 360W | Found on: Amazon.com Brand:ALITOVE | the main power source of the machine. Input Voltage: 220 Volts Output: DC 12V 30A max. | |
| PTFE Tubing | Found on Amazon.com | ||
| RITEFLOW FLOWMETERS WITH PLAIN ENDS (UNMOUNTED) | Found on:Globalindustrial.com Model number:t9FB3075514 | Item number: H40407-0075 | Monitors air flow in the olfactometer 150mm Scale, Size 2 Manufacturers Part Number:H40407-0075 |
| Screw driver | Found on: Amazon.com Brand:Sharden | Used for screws on olfactometer | |
| Shrimk wrap/tubing(various sizes to fir 18-22 gauge wire | Found on: Amazon.com Brand:eventronic | Material:Made of Polyolefin, Shrinkage Ratio:2:1 (will shrink to 1/2 its supplied diameter) | |
| Silicone Tubing 0.030 x 0.065 | Found on:Amazon.com Brand:Scientific commodities | Tubing for the odor vials | |
| Solder- with lead | Found on:Uline.com | S-25294 | Will be used with the soldering iron |
| Soldering iron | Found on:Uline.com | Model NO. H-10799 | Will be used to solder the Bottons on the control box and other connections |
| Solid State Relay Module Quad Output – Red | Found on: https://www.sealevel.com/ | Part: OB5Q Model: DC Output QSSR Module | Capacitance: 8 pF Dimensions: 2.4" (L) x 1.1" (W) x 3.1" (H) # of I/O : 4 Outputs Max Line Voltage: 60 VDC Max On-State Current: 3A Minimum Line Voltage: 3 VDC Operating Temperature :-30°C to 80°C (-22°F to 176°F) Output Isolation: 4000 Vrms Storage Temperature: -40°C to 100°C (-40°F to 212°F) |
| SPST pushbutton switch | Brand:Apiele Found on: Amazon.com | n/a | Used inside of the control box control water valve and final valve. Operating voltage 250v Current rating: 1 Amps, 3 Amps |
| Ssr-rack 48 | Found on:Radwell.com | SKU: 83105002 | Processes all the connections of the olfactometer and works with quad-type sooid state relays. |
| Stainless steel feeding tubes | |||
| Tip tinner and cleaner | Found on: Amazon.com Brand:Thermaltronics | Model number: FBA-TMT-TC-2 | |
| Valve Ball PVC 1/4” barb buna | Found on: uplastic.com | Item number:62281 | |
| water valve | Found on: Ph.parker.com | Part #: 003-0257-900 | Pumps water into the chamber Maximum Flow Rate: 1500 sccm Voltage (VDC): 24 Maximum Operating Pressure:50 psi, 3.44 bar |
| Wire 22awg | Brand:tuofeng Found on: Amazon.com | N/a | Used to wire different components of the olfactometer Material:copper Gauge 30.0 |
| Wire snips | Found on:Amazon.com Brand:Billbotk | Will be used to snip wires | |
| As Brand: PTFE | Part #036663601452 | Use for delivering odorants. | |
| https://medschool.cuanschutz.edu/neurotechnologycenter/Cores/machine-shop |
References
- Yeshurun, Y., Sobel, N. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu Rev Psychol. 61, 219-241 (2010).
- Buck, L. B. Unraveling the sense of smell (Nobel lecture). Angew Chem Int Ed Engl. 44, 6128-6140 (2005).
- Spors, H., Wachowiak, M., Cohen, L. B., Friedrich, R. W. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 26 (4), 1247-1259 (2006).
- Feinstein, P., Bozza, T., Rodriguez, I., Vassalli, A., Mombaerts, P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 117 (6), 833-846 (2004).
- Soucy, E. R., Albeanu, D. F., Fantana, A. L., Murthy, V. N., Meister, M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 12, 210-220 (2009).
- Spors, H., et al. Illuminating vertebrate olfactory processing. J Neurosci. 32, 14102-14108 (2012).
- Kwak, J., Willse, A., Preti, G., Yamazaki, K., Beauchamp, G. K. In search of the chemical basis for MHC odourtypes. Proc Biol Sci. 277 (1693), 2417-2425 (2010).
- Schaefer, M. L., Yamazaki, K., Osada, K., Restrepo, D., Beauchamp, G. K. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 22 (21), 9513-9521 (2002).
- Li, Y., et al. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat Neurosci. 20 (4), 559-570 (2017).
- Gadziola, M. A., et al. A neural system that represents the association of odors with rewarded outcomes and promotes behavioral engagement. Cell Rep. 32 (3), 107919(2020).
- Carlson, K. S., Gadziola, M. A., Dauster, E. S., Wesson, D. W. Selective attention controls olfactory decisions and the neural encoding of odors. Curr Biol. 28 (14), 2195-2205.e4 (2018).
- Moberly, A. H., et al. Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat Commun. 9, 1528(2018).
- Gire, D. H., et al. Information for decision-making and stimulus identification is multiplexed in sensory cortex. Nat Neurosci. 16 (8), 991-993 (2013).
- Ma, M., et al. Molecular layer interneurons in the cerebellum encode for valence in associative learning. Nat Commun. 11 (1), 4217(2020).
- Bolding, K. A., et al. Robust odor coding across states in piriform cortex requires recurrent circuitry: evidence for pattern completion in an associative network. bioRxiv. , 694331(2019).
- Bolding, K. A., Franks, K. M. Recurrent cortical circuits implement concentration-invariant odor coding. Science. 361 (6407), eaat6904(2018).
- Bolding, K. A., Franks, K. M. Complementary codes for odor identity and intensity in olfactory cortex. Elife. 6, e22630(2017).
- Slotnick, B., Restrepo, D. Olfactometry with mice. Curr Protoc Neurosci. Chapter 8 (Unit 8.20), (2005).
- Williams, E., Dewan, A. Olfactory detection thresholds for primary aliphatic alcohols in mice. Chem Senses. 45 (7), 513-521 (2020).
- Arevalo, N., et al. Open-source JL olfactometer for awake behaving recording of brain activity for mice engaged in olfactory tasks. Animal Models of Reproductive Behavior. Neuromethods. 200, (2023).
- Slotnick, B., Bodyak, N. Odor discrimination and odor quality perception in rats with disruption of connections between the olfactory epithelium and olfactory bulbs. J Neurosci. 22 (10), 4205-4216 (2002).
- Burton, S. D., et al. A novel olfactometer for efficient and flexible odorant delivery. Chem Senses. 44 (3), 173-188 (2019).
- Slotnick, B., Cockerham, R., Pickett, E. Olfaction in olfactory bulbectomized rats. J Neurosci. 24 (41), 9195-9200 (2004).
- Reinert, J. K., Schaefer, A. T., Kuner, T. High-throughput automated olfactory phenotyping of group-housed mice. Front Behav Neurosci. 13, 267(2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved