Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Method Article
Producción de masa fundida de vidrio sintético Nuclear
En este artículo
Resumen
A protocol for the production of synthetic nuclear melt glass, similar to trinitite, is presented.
Resumen
Realistic surrogate nuclear debris is needed within the nuclear forensics community to test and validate post-detonation analysis techniques. Here we outline a novel process for producing bulk surface debris using a high temperature furnace. The material developed in this study is physically and chemically similar to trinitite (the melt glass produced by the first nuclear test). This synthetic nuclear melt glass is assumed to be similar to the vitrified material produced near the epicenter (ground zero) of any surface nuclear detonation in a desert environment. The process outlined here can be applied to produce other types of nuclear melt glass including that likely to be formed in an urban environment. This can be accomplished by simply modifying the precursor matrix to which this production process is applied. The melt glass produced in this study has been analyzed and compared to trinitite, revealing a comparable crystalline morphology, physical structure, void fraction, and chemical composition.
Introducción
Concerns over the potential malicious use of nuclear weapons by terrorists or rogue nations have highlighted the importance of nuclear forensics analysis for the purpose of attribution.1 Rapid post-detonation analysis techniques are desirable to shorten the attribution timeline as much as possible. The development and validation of such techniques requires realistic nuclear debris samples for testing. Nuclear testing no longer occurs in the United States and nuclear surface debris from the testing era is not readily available (with the exception of trinitite - the melt glass produced by the first nuclear test at the trinity site) and therefore realistic surrogate debris is needed.
The primary goal of the method described here is the production of realistic surrogate nuclear debris similar to trinitite. Synthetic nuclear melt glass samples which are readily available to the academic community can be used to test existing analysis techniques and to develop new methods such as thermo-chromatography for rapid post-detonation analysis.2 With this goal in mind the current study is focused on producing samples which mimic trinitite but do not contain any sensitive weapon design information. The fuel and tamper components within these samples are completely generic and the comparison to trinitite is based on chemistry, morphology, and physical characteristics. The similarities between trinitite and the synthetic nuclear melt glass produced in this study have been previously discussed.3
The purpose of this article is to outline the details of the production process used at the University of Tennessee (UT). This production process was developed with two key parameters in mind: 1) the composition of material incorporated into nuclear melt glass, and 2) the melting temperature of the material. Methods exist for estimating the melting temperature of glass forming networks4 and these techniques have been employed here, along with additional experimentation to determine the optimal processing temperature for the trinitite matrix.5
Alternative methods for surrogate debris production have been published recently. The use of high power lasers has the advantage of creating sufficiently high temperatures to cause elemental fractionation within the target matrix.6 Porous chromatographic substrates have been used to produce small particles similar to fallout particles using condensed phase methods7. The latter method is most useful for producing particulate debris (nuclear fallout) and has been demonstrated with natural metals. The advantages of the method presented here are 1) simplicity, 2) reproducibility, and 3) scalability (sample sizes can range from tiny beads to large chunks of melt glass). Also, this method is expandable both in terms of production output and variety of explosive scenarios covered, and it has already been demonstrated using radioactive materials. A sample has been successfully activated at the High Flux Isotope Reactor (HFIR) at Oak Ridge National Laboratory (ORNL). Natural uranium compounds were added to the matrix prior to melting and fission products were produced in situ by neutron irradiation.
Methods within the glass making industry and those employed for the purpose of radioactive waste immobilization8 have been consulted in the development of the method presented here. The unique effects of radiation in glasses are of inherent interest9 and will constitute an important area of study as this method is further developed.
The method described below is appropriate for any application where a bulk melt glass sample is desired. These samples most closely resemble the material found near the epicenter of a nuclear explosion. Samples of various sizes can be produced, however, methods employing plasma torches or lasers will be more useful for simulating fine particulate debris. Also, commercial HTFs do not reach temperatures high enough to cause elemental fractionation for a wide range of elements. This method should be employed when physical and morphological characteristics are of primary importance.
Protocolo
Precaución: El proceso descrito aquí incluye el uso de material radiactivo (por ejemplo, hexahidrato de nitrato de uranio) y varias sustancias corrosivas. Ropa de protección adecuada y el equipo deben ser utilizados (incluyendo una bata de laboratorio, guantes, protección para los ojos, y una campana de humos) durante la preparación de la muestra. Además, las áreas de laboratorio utilizados para este trabajo deben ser controlados regularmente por la contaminación radiactiva.
Nota:. Los compuestos químicos necesarios se enumeran en la Tabla 1 Esta formulación se desarrolló mediante el examen previamente reportó datos de composición para trinitita 10 Las fracciones de masa aquí presentados fueron determinados por un promedio de las fracciones de masa de varias muestras trinitita diferentes 10 La masa "desaparecidos".. (las fracciones que no suman a la unidad) existe para permitir cierta flexibilidad a la hora de agregar combustible, sabotaje, y otros componentes. Nuestro análisis independiente de varias muestras trinitita sugiere que el cuarzo es la única fase mineralsobrevivir en trinitita. 5 Por lo tanto, el cuarzo es el único mineral incluido en nuestra trinitita formulación estándar (STF). Aunque granos reliquia de otros minerales han sido reportados en trinitita, 11 estos tienden a ser la excepción, no la regla. En general, el cuarzo es el único mineral que se encuentra en el vidrio fundido. 10,12 Además, la arena de cuarzo es un componente común de asfalto y hormigón que será importante en la formación de la masa fundida de vidrio nuclear urbana.
-4| Promedió datos trinitita | Estándar trinitita Formulación (STF) | ||
| Compuesto | Fracción de masa | Compuesto | Fracción de masa |
| SiO 2 | 6.42x10 -1 | SiO 2 | 6.42x10 -1 |
| Al 2 O 3 | 1.43x10 -1 | Al 2 O 3 | 1.43x10 -1 |
| CaO | 9.64x10 -2 | CaO | 9.64x10 -2 |
| FeO | 1.97x10 -2 | 1.97x10 -2 | |
| MgO | 1.15x10 -2 | MgO | 1.15x10 -2 |
| Na2O | 1.25x10 -2 | Na2O | 1.25x10 -2 |
| K 2 O | 5.13x10 -2 | KOH | 6.12x10 -2 |
| MnO | 5.05x10 -4 | MnO | |
| TiO 2 | 4.27x10 -3 | TiO 2 | 4.27x10 -3 |
| Total | 9.81x10 -1 | Total | 9.91x10 -1 |
Tabla 1. Lista de compuestos químicos.
1. Preparación de la STF
Nota: El equipo necesario incluye una microbalanza, espátulas de metal, un mortero y una maja de cerámica, una campana de humos químicos, guantes de látex, una bata de laboratorio y protección para los ojos.
- La mezcla de componentes no radiactivos
- Adquirir al menos 65 g de arena de cuarzo (SiO2), 15 g de Al 2 O 3 </ sub> en polvo, 10 g de polvo de CaO, 2 g de polvo de FeO, 2 g de polvo de MgO, 2 g de Na 2 polvo de O, 7 g de gránulos de KOH, 1 g de polvo de MnO y 1 g de TiO 2 en polvo ( compuestos enumerados en la Tabla 1).
- Utilice una microbalanza y pequeña espátula para medir con precisión las fracciones de masa de cada compuesto enumerados en la Tabla 1. Para obtener los mejores resultados preparan 100 g de la matriz de precursor no radiactivo al mismo tiempo.
- Use un mortero para pulverizar (a ~ 10-20 gránulos de tamaño de micras) ya fondo mezclar los compuestos, formando una mezcla en polvo homogénea que contiene 64,2 g de SiO2, 14,2 g de Al 2 O 3, 9.64 g de CaO, 1,97 g de FeO, 1,15 g de MgO, 1,25 g de Na 2 O. 6,12 g de KOH, 0,0505 g de MnO, y 0,427 g de TiO 2.
- Agitar la mezcla, utilizando un mezclador de bola, poco antes de que se tome la siguiente etapa.
- La mezcla del STF con uranio nitrato hexahidrato (UNH)
- Acquire al menos 1 g de UNH.
- Dentro de una campana de humos, pulverizar unos pocos cristales UNH (utilizando un mortero y almirez) para formar un polvo fino de 1-2 micras gránulos.
- Añadir 33.75 g de UNH por gramo de la matriz de precursor no radiactivo (esta relación es apropiado para la simulación de un arma simple con un rendimiento de 1 kilotones). 13
- Mezclar bien la mezcla de polvo, incluyendo la UNH, utilizando un mortero. Completar mezcla final poco antes de la etapa de fusión.
2. La producción de 1 gramo Melt Glass Muestra
Nota: El equipo necesario incluye una HTF nominal de 1.600 ° C o superior, crisoles de alta pureza de grafito, acero inoxidable largas pinzas, guantes resistentes al calor y protección para los ojos. Guantes resistentes al calor y protección para los ojos se deben usar al introducir o extraer muestras del horno. Gafas de seguridad tintados (o gafas de sol) son útiles, ya que reducen el resplandor del horno.
- Producción de una muestra no radiactivo
- Llenar un plato de cerámica gruesa (tal como un mortero) con ~ 100 g de arena de cuarzo puro y mantener a temperatura ambiente cerca de la ubicación del horno donde se fundieron las muestras.
- Precaliente el HTF a 1500 ° C.
- Mida cuidadosamente 1,00 g de la mezcla de polvo no radiactivo y colocar el polvo en un crisol de grafito de alta pureza.
- Coloque cuidadosamente el crisol en el HTF climatizada (con un largo par de pinzas de crisol de acero) y derretir la mezcla durante 30 min.
- Retire la muestra (de nuevo utilizando las pinzas) y verter la muestra fundida en el mortero lleno de arena.
- Deje que la cuenta de vidrio se enfríe durante 1-2 minutos antes de manipular.
- Pulir el grano para eliminar la arena residual (si es necesario).
- Producción de una muestra radiactiva
- Repita los pasos 2.1.1 y 2.1.2.
- Mida cuidadosamente 1,00 gramo de la mezcla de polvo radiactivo (incluyendo UNH) y colocar el powd er en un crisol de grafito de alta pureza con una espátula y microbalanza separada para evitar la contaminación cruzada.
- Repita los pasos 2.1.4 - 2.1.6 arriba.
- Monitorear el área alrededor del horno (usando un detector de mano de radiación y / o ensayos de banda magnética) para comprobar si hay contaminación radiactiva.
Activación 3. Muestra
Nota: Las ecuaciones que siguen se obtuvieron asumiendo el uso de armas de grado (enriquecido) uranio metálico. Tendrá que ser reducido de acuerdo con la fracción de masa de uranio elemental y el nivel de enriquecimiento de U 235 Las cantidades de UNH u óxido de uranio.
- La activación de una muestra del derretimiento de vidrio con uranio Fue
- Calcular la fracción de masa de uranio metálico requerido para la muestra usando la siguiente ecuación 13 (donde m U representa la fracción de masa de uranio y Y representa el rendimiento arma):
473 / 53473eq1.jpg "/> - Opcional: Se calcula la fracción de masa de manipulación indebida (por ejemplo, el uranio natural, plomo, tungsteno) utilizando la siguiente ecuación: 13
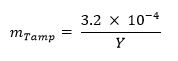
- Calcular el número objetivo de fisiones en la muestra usando la siguiente ecuación 13 donde M s representa la masa de la muestra en gramos y N f representa el número de fisiones que se producen en la muestra durante la irradiación:
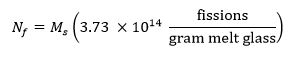
- Calcular el tiempo de irradiación necesario utilizando la siguiente ecuación 13 donde m 235 representa la fracción de masa 235 U (nivel de enriquecimiento) y t TIR es el tiempo de irradiación en segundos:
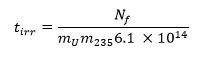
- Irradiar la muestra paraT segundos IRR a un flujo de neutrones térmicos de 4,0 x 10 14 n / cm 2 / s. Por ejemplo, una irradiación de 60 seg en Pneumatic Tubo 1 (PT-1) en HFIR (con una térmica a la resonancia relación de 35) producirá aproximadamente 1,1 x 10 11 fisiones en una muestra que contiene 870 g de UNH (equivalente a 410 mg de uranio natural, o 3,0 g de 235 U). Esto se ha logrado por una perla de vidrio 0,433 g diseñado para simular una muestra de vidrio de fusión producida por un arma con un rendimiento 0,1 kilotones. Esta muestra se ha analizado a fondo por Cook et al. 14
- Siga los protocolos de seguridad vigentes para el manejo de la muestra radiactiva después de la irradiación.
- Calcular la fracción de masa de uranio metálico requerido para la muestra usando la siguiente ecuación 13 (donde m U representa la fracción de masa de uranio y Y representa el rendimiento arma):
- La activación de una muestra del derretimiento de cristal con plutonio de combustible (Factores de Planificación)
- Calcular la fracción de masa de plutonio metal requerido para la muestra usando la siguiente ecuación 13 donde m represen Puts la fracción de masa de plutonio e Y representa el rendimiento arma:
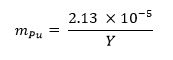
- Repita los pasos 3.1.2 y 3.1.3 anteriores.
- Determinar el tiempo de irradiación necesario para obtener el número deseado de fisiones en la muestra de vidrio fundido. Este tiempo dependerá de la composición y el grado del plutonio, así como el espectro de energía de neutrones.
- Calcular la fracción de masa de plutonio metal requerido para la muestra usando la siguiente ecuación 13 donde m represen Puts la fracción de masa de plutonio e Y representa el rendimiento arma:
Nota: Gran se debe tener cuidado cuando se trata de plutonio y análisis adicional será requerida. Al escribir estas líneas, sólo el uranio se ha utilizado en las muestras de vidrio de fusión sintéticos producidos en UT y irradiados en HFIR.
Resultados
Las muestras no radiactivos producidos en este estudio han sido comparados con trinitita y las Figuras 1-3 muestran que las propiedades físicas y la morfología son, en efecto similar. La Figura 1 proporciona fotografías que revelan las similitudes en color y textura que se observan a nivel macroscópico. La figura 2 muestra microscopio electrónico de barrido (SEM) Imágenes secundarias Electron (SE) que revelan características similares a nivel mic...
Discusión
Nota respecto a los pasos 1.2.2 y 1.2.3: La cantidad exacta de UNH variará dependiendo del escenario que se está simulando. Las fórmulas de planificación desarrollados por Giminaro et al. Se pueden utilizar para elegir la masa adecuada de uranio para una muestra dada 13 como se explica en la sección "Activación de ejemplo" de este artículo. También, óxido de uranio (UO 2 o U 3 O 8) se puede utilizar en lugar de UNH, si está disponible, y la fracci?...
Divulgaciones
This work was performed under grant number DE-NA0001983 from the Stewardship Science Academic Alliances (SSAA) Program of the National Nuclear Security Administration (NNSA).
Agradecimientos
Portions of this study have been previously published in the Journal of Radioanalytical and Nuclear Chemistry.3,13 A patent is pending for this method.
Materiales
| Name | Company | Catalog Number | Comments |
| High Temperature Furnace (HTF) | Carbolite | HTF 18 | 1,800 °C HTF used to melt samples |
| High Temperature Drop Furnace | CM Inc. | 1706 BL | 1,700 °C Drop Furnace used to melt samples |
| Graphite Crucibles | SCP Science | 040-060-041 | 27 ml high purity graphite crucibles (10 pack) |
| Crucible Tongs | Grainger | 5ZPV0 | 26 in., stainless steele tongs for handling crucibles |
| Heat Resistent Gloves | Grainger | 8814-09 | Gloves used to protect hands from heat during sample intro/removal |
| Mortar & Pestle | Fisherbrand | S337631 | 300 ml, Ceramic mortar and pestle for powdering and mixing |
| Micro Balance | Grainger | 8NJG2 | 220 g Cap, high precision scale for measuring powder mass |
| Spatulas | Fisherbrand | 14374 | Metal spatulas for measure small quantities of powder |
| SiO2 | Sigma-Aldrich | 274739-5KG | Quartz Sand CAS Number: 14808-60-7 |
| Al2O3 | Sigma-Aldrich | 11028-1KG | Aluminum Oxide Powder CAS Number: 1344-28-1 |
| CaO | Sigma-Aldrich | 12047-2.5KG | Calcium Oxide Powder CAS Number: 1305-78-8 |
| FeO | Sigma-Aldrich | 400866-25G | Iron Oxide Powder CAS Number: 1345-25-1 |
| MgO | Sigma-Aldrich | 342793-250G | Magnesium Oxide Powder CAS Number: 1309-48-4 |
| Na2O | Sigma-Aldrich | 36712-25G | Sodium Oxide Powder CAS Number: 1313-59-3 |
| KOH | Sigma-Aldrich | 278904-250G | Potasium Hydroxide Pellets CAS Number: 12030-88-5 |
| MnO | Sigma-Aldrich | 377201-500G | Manganese Oxide Powder CAS Number: 1344-43-0 |
| TiO2 | Sigma-Aldrich | 791326-5G | Titanium Oxide Beads CAS Number: 12188-41-9 |
Referencias
- Carnesdale, A. . Nuclear Forensics: A Capability at Risk (Abbreviated Version). , (2010).
- Garrison, J. R., Hanson, D. E., Hall, H. L. Monte Carlo analysis of thermochromatography as a fast separation method for nuclear forensics. J Radioanal Nucl Chem. 291 (3), 885-894 (2011).
- Molgaard, J. J., et al. Development of synthetic nuclear melt glass for forensic analysis. J Radioanal Nucl Chem. 304 (3), 1293-1301 (2015).
- Fluegel, A. Modeling of Glass Liquidus Temperatures using Disconnected Peak Functions. , (2007).
- Oldham, C. J., Molgaard, J. J., Auxier, J. D., Hall, H. L. Comparison of Nuclear Debris Surrogates Using Powder X-Ray Diffraction. , (2014).
- Liezers, M., Fahey, A. J., Carman, A. J., Eiden, G. C. The formation of trinitite-like surrogate nuclear explosion debris ( SNED ) and extreme thermal fractionation of SRM-612 glass induced by high power CW CO 2 laser irradiation. J Radional Nucl Chem. 304 (2), 705-715 (2015).
- Harvey, S. D., et al. Porous chromatographic materials as substrates for preparing synthetic nuclear explosion debris particles. J Radioanal Nucl Chem. 298 (3), 1885-1898 (2013).
- Hanni, J. B., et al. Liquidus temperature measurements for modeling oxide glass systems relevant to nuclear waste vitrification. J Mater Res. 20 (12), 3346-3357 (2005).
- Weber, W. J., et al. Radiation Effects in Glasses Used for Immobilization of High-Level Waste and Plutonium Disposition. J Mater Res. 12 (8), 1946-1978 (1997).
- Eby, N., Hermes, R., Charnley, N., Smoliga, J. A. Trinitite-the atomic rock. Geol Today. 26 (5), 180-185 (2010).
- Bellucci, J. J., Simonetti, A. Nuclear forensics: searching for nuclear device debris in trinitite-hosted inclusions. J Radioanal Nucl Chem. 293 (1), 313-319 (2012).
- Ross, C. S. . Optical Properties of Glass from Alamogordo, New Mexico. , (1948).
- Giminaro, A. V., et al. Compositional planning for development of synthetic urban nuclear melt glass. J Radional Nucl Chem. , (2015).
- Cook, M. T., Auxier, J. D., Giminaro, A. V., Molgaard, J. J., Knowles, J. R., Hall, H. L. A comparison of gamma spectra from trinitite versus irradiated synthetic nuclear melt glass. J Radioanal Nucl Chem. , (2015).
- Fahey, J., Zeissler, C. J., Newbury, D. E., Davis, J., Lindstrom, R. M. Postdetonation nuclear debris for attribution. Proc Natl Acad Sci U S A. 107 (47), 20207-20212 (2010).
- Bellucci, J. J., Simonetti, A., Koeman, E. C., Wallace, C., Burns, P. C. A detailed geochemical investigation of post-nuclear detonation trinitite glass at high spatial resolution: Delineating anthropogenic vs. natural components. Chem Geol. 365, 69-86 (2014).
- Donohue, P. H., Simonetti, A., Koeman, E. C., Mana, S., Peter, C. Nuclear Forensic Applications Involving High Spatial Resolution Analysis of Trinitite Cross-Sections. J Radioanal Nucl Chem. , (2015).
- Eaton, G. F., Smith, D. K. Aged nuclear explosive melt glass: Radiography and scanning electron microscope analyses documenting radionuclide distribution and glass alteration. J Radioanal Nucl Chem. 248 (3), 543-547 (2001).
- Kersting, A. B., Smith, D. K. . Observations of Nuclear Explosive Melt Glass Textures and Surface Areas. , (2006).
- . . IAEA Safeguards Glossary. , (2001).
- Glasstone, S., Dolan, P. . Effects of Nuclear Weapons. , (1977).
- Carney, K. P., Finck, M. R., McGrath, C. A., Martin, L. R., Lewis, R. R. The development of radioactive glass surrogates for fallout debris. J Radioanal Nucl Chem. 299 (1), 363-372 (2013).
- Molgaard, J. J., Auxier, J. D., Hall, H. L. A Comparison of Activation Products in Different Types of Urban Nuclear Melt Glass. , (2015).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados