A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Production of Synthetic Nuclear Melt Glass
In This Article
Summary
A protocol for the production of synthetic nuclear melt glass, similar to trinitite, is presented.
Abstract
Realistic surrogate nuclear debris is needed within the nuclear forensics community to test and validate post-detonation analysis techniques. Here we outline a novel process for producing bulk surface debris using a high temperature furnace. The material developed in this study is physically and chemically similar to trinitite (the melt glass produced by the first nuclear test). This synthetic nuclear melt glass is assumed to be similar to the vitrified material produced near the epicenter (ground zero) of any surface nuclear detonation in a desert environment. The process outlined here can be applied to produce other types of nuclear melt glass including that likely to be formed in an urban environment. This can be accomplished by simply modifying the precursor matrix to which this production process is applied. The melt glass produced in this study has been analyzed and compared to trinitite, revealing a comparable crystalline morphology, physical structure, void fraction, and chemical composition.
Introduction
Concerns over the potential malicious use of nuclear weapons by terrorists or rogue nations have highlighted the importance of nuclear forensics analysis for the purpose of attribution.1 Rapid post-detonation analysis techniques are desirable to shorten the attribution timeline as much as possible. The development and validation of such techniques requires realistic nuclear debris samples for testing. Nuclear testing no longer occurs in the United States and nuclear surface debris from the testing era is not readily available (with the exception of trinitite - the melt glass produced by the first nuclear test at the trinity site) and therefore realistic surrogate debris is needed.
The primary goal of the method described here is the production of realistic surrogate nuclear debris similar to trinitite. Synthetic nuclear melt glass samples which are readily available to the academic community can be used to test existing analysis techniques and to develop new methods such as thermo-chromatography for rapid post-detonation analysis.2 With this goal in mind the current study is focused on producing samples which mimic trinitite but do not contain any sensitive weapon design information. The fuel and tamper components within these samples are completely generic and the comparison to trinitite is based on chemistry, morphology, and physical characteristics. The similarities between trinitite and the synthetic nuclear melt glass produced in this study have been previously discussed.3
The purpose of this article is to outline the details of the production process used at the University of Tennessee (UT). This production process was developed with two key parameters in mind: 1) the composition of material incorporated into nuclear melt glass, and 2) the melting temperature of the material. Methods exist for estimating the melting temperature of glass forming networks4 and these techniques have been employed here, along with additional experimentation to determine the optimal processing temperature for the trinitite matrix.5
Alternative methods for surrogate debris production have been published recently. The use of high power lasers has the advantage of creating sufficiently high temperatures to cause elemental fractionation within the target matrix.6 Porous chromatographic substrates have been used to produce small particles similar to fallout particles using condensed phase methods7. The latter method is most useful for producing particulate debris (nuclear fallout) and has been demonstrated with natural metals. The advantages of the method presented here are 1) simplicity, 2) reproducibility, and 3) scalability (sample sizes can range from tiny beads to large chunks of melt glass). Also, this method is expandable both in terms of production output and variety of explosive scenarios covered, and it has already been demonstrated using radioactive materials. A sample has been successfully activated at the High Flux Isotope Reactor (HFIR) at Oak Ridge National Laboratory (ORNL). Natural uranium compounds were added to the matrix prior to melting and fission products were produced in situ by neutron irradiation.
Methods within the glass making industry and those employed for the purpose of radioactive waste immobilization8 have been consulted in the development of the method presented here. The unique effects of radiation in glasses are of inherent interest9 and will constitute an important area of study as this method is further developed.
The method described below is appropriate for any application where a bulk melt glass sample is desired. These samples most closely resemble the material found near the epicenter of a nuclear explosion. Samples of various sizes can be produced, however, methods employing plasma torches or lasers will be more useful for simulating fine particulate debris. Also, commercial HTFs do not reach temperatures high enough to cause elemental fractionation for a wide range of elements. This method should be employed when physical and morphological characteristics are of primary importance.
Protocol
Caution: The process outlined here includes the use of radioactive material (e.g., Uranium Nitrate Hexahydrate) and several corrosive substances. Appropriate protective clothing and equipment should be used (including a lab coat, gloves, eye protection, and a fume hood) during sample preparation. In addition, laboratory areas used for this work should be monitored regularly for radioactive contamination.
Note: The chemical compounds needed are listed in Table 1. This formulation was developed by examining previously reported compositional data for trinitite.10 The mass fractions reported here were determined by averaging the mass fractions for several different trinitite samples.10 The "missing" mass (the fractions to not sum to unity) exists to allow for some flexibility when adding fuel, tamper, and other constituents. Our independent analysis of several trinitite samples suggests that quartz is the only mineral phase surviving in trinitite.5 Therefore, quartz is the only mineral included in our Standard Trinitite Formulation (STF). Although relic grains of other minerals have been reported in trinitite,11 these tend to be the exception, rather than the rule. In general, quartz is the only mineral found in the melt glass.10,12 Also, quartz sand is a common component of asphalt and concrete which will be important in the formation of urban nuclear melt glass.
| Averaged Trinitite Data | Standard Trinitite Formulation (STF) | ||
| Compound | Mass Fraction | Compound | Mass Fraction |
| SiO2 | 6.42x10-1 | SiO2 | 6.42x10-1 |
| Al2O3 | 1.43x10-1 | Al2O3 | 1.43x10-1 |
| CaO | 9.64x10-2 | CaO | 9.64x10-2 |
| FeO | 1.97x10-2 | FeO | 1.97x10-2 |
| MgO | 1.15x10-2 | MgO | 1.15x10-2 |
| Na2O | 1.25x10-2 | Na2O | 1.25x10-2 |
| K2O | 5.13x10-2 | KOH | 6.12x10-2 |
| MnO | 5.05x10-4 | MnO | 5.05x10-4 |
| TiO2 | 4.27x10-3 | TiO2 | 4.27x10-3 |
| Total | 9.81x10-1 | Total | 9.91x10-1 |
Table 1. List of chemical compounds.
1. Preparation of the STF
Note: Equipment needed includes a microbalance, metal spatulas, a ceramic mortar and pestle, a chemical fume hood, latex gloves, a lab coat, and eye protection.
- Mixing of non-radioactive components
- Acquire at least 65 g of quartz sand (SiO2), 15 g of Al2O3 powder, 10 g of CaO powder, 2 g of FeO powder, 2 g of MgO powder, 2 g of Na2O powder, 7 g of KOH pellets, 1 g of MnO powder and 1 g of TiO2 powder (compounds listed in Table 1).
- Use a microbalance and small spatula to precisely measure the mass fractions of each compound as listed in Table 1. For best results prepare 100 g of the non-radioactive precursor matrix at one time.
- Use a mortar and pestle to pulverize (to ~10-20 µm size granules) and thoroughly mix the compounds, forming a homogenous powder mixture containing 64.2 g of SiO2, 14.2 g of Al2O3, 9.64 g of CaO, 1.97 g of FeO, 1.15 g of MgO, 1.25 g of Na2O. 6.12 g of KOH, 0.0505 g of MnO, and 0.427 g of TiO2.
- Agitate the mixture, using a ball mixer, shortly before the next step is taken.
- Mixing of STF with Uranium Nitrate Hexahydrate (UNH)
- Acquire at least 1 g of UNH.
- Inside a fume hood, pulverize a few UNH crystals (using a mortar and pestle) to form a fine powder of 1-2 µm granules.
- Add 33.75 µg of UNH per gram of the non-radioactive precursor matrix (this ratio is appropriate for simulating a simple weapon with a yield of 1 kiloton).13
- Thoroughly mix the powder mixture, including the UNH, using a mortar and pestle. Complete final mixing shortly before the melting step.
2. Production of 1-gram Melt Glass Sample
Note: Equipment needed includes an HTF rated at 1,600 °C or higher, high purity graphite crucibles, long stainless steel crucible tongs, heat resistant gloves, and eye protection. Heat resistant gloves and eye protection should be worn when introducing or removing samples from the furnace. Tinted safety goggles (or sun glasses) are useful as they reduce the glare from the furnace.
- Production of a non-radioactive sample
- Fill a thick ceramic dish (such as a mortar) with ~100 g of pure quartz sand and maintain at RT near the location of the furnace where the samples will be melted.
- Preheat the HTF to 1,500 °C.
- Carefully measure 1.00 g of the non-radioactive powder mixture and place the powder in a high purity graphite crucible.
- Carefully place the crucible in the heated HTF (using a long pair of steel crucible tongs) and melt the mixture for 30 min.
- Remove the sample (again using the tongs) and pour the molten sample into the mortar filled with sand.
- Allow the glass bead to cool for 1-2 min before handling.
- Polish the bead to remove residual sand (if necessary).
- Production of a radioactive sample
- Repeat steps 2.1.1 and 2.1.2 above.
- Carefully measure 1.00 gram of the radioactive powder mixture (including UNH) and place the powder in a high purity graphite crucible using a separate spatula and microbalance to avoid cross contamination.
- Repeat steps 2.1.4 - 2.1.6 above.
- Monitor the area around the furnace (using a hand-held radiation detector and/or swipe assays) to check for radioactive contamination.
3. Sample Activation
Note: The equations that follow were derived assuming the use of weapons grade (enriched) uranium metal. The quantities of UNH or Uranium Oxide will need to be scaled according to the mass fraction of elemental uranium and the level of 235U enrichment.
- Activation of a Melt Glass Sample with Uranium Fue
- Calculate the mass fraction of uranium metal required for the sample using the equation below13 (where mU represents the uranium mass fraction and Y represents the weapon yield):
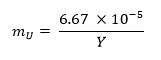
- Optional: Calculate the mass fraction of tamper (e.g., natural uranium, lead, tungsten) using the equation below:13
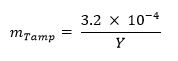
- Calculate the target number of fissions in the sample using the following equation13 where Ms represents the mass of the sample in grams and Nf represents the number of fissions produced in the sample during irradiation:
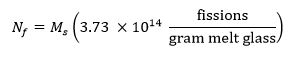
- Calculate the required irradiation time using the equation below13 where m235 represents the 235U mass fraction (enrichment level) and tirr is the irradiation time in seconds:
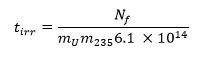
- Irradiate the sample for tirr seconds at a thermal neutron flux of 4.0 x 1014 n/cm2/sec. For example, a 60 sec irradiation in Pneumatic Tube 1 (PT-1) at HFIR (with a thermal to resonance ratio of 35) will produce approximately 1.1 x 1011 fissions in a sample containing 870 µg of UNH (equivalent to 410 µg of natural uranium, or 3.0 µg of 235U). This has been accomplished for one 0.433 g glass bead designed to simulate a melt glass sample produced by a weapon with a 0.1 kiloton yield. This sample has been thoroughly analyzed by Cook et al.14
- Follow applicable safety protocols for handling the radioactive sample post-irradiation.
- Calculate the mass fraction of uranium metal required for the sample using the equation below13 (where mU represents the uranium mass fraction and Y represents the weapon yield):
- Activation of a Melt Glass Sample with Plutonium Fuel (Planning Factors)
- Calculate the mass fraction of plutonium metal required for the sample using the equation below13 where mPu represents the plutonium mass fraction and Y represents the weapon yield:
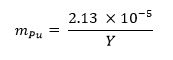
- Repeat Steps 3.1.2 and 3.1.3 above.
- Determine the irradiation time required to obtain the desired number of fissions in the melt glass sample. This time will depend on the composition and grade of the plutonium as well as the neutron energy spectrum.
- Calculate the mass fraction of plutonium metal required for the sample using the equation below13 where mPu represents the plutonium mass fraction and Y represents the weapon yield:
Note: Great care should be taken when dealing with plutonium and additional analysis will be required. As of this writing, only uranium has been used in the synthetic melt glass samples produced at UT and irradiated at HFIR.
Results
The non-radioactive samples produced in this study have been compared to trinitite and Figures 1-3 show that the physical properties and morphology are indeed similar. Figure 1 provides photographs that reveal the similarities in color and texture which are observed at the macroscopic level. Figure 2 shows Scanning Electron Microscope (SEM) Secondary Electron (SE) images which reveal similar features at the micron level. SEM analysis was performed using a SEM and SEM sof...
Discussion
Note regarding steps 1.2.2 and 1.2.3: The exact amount of UNH will vary depending on the scenario being simulated. The planning formulas developed by Giminaro et al. can be used to choose the appropriate mass of uranium for a given sample13 as discussed in the "Sample Activation" section of this paper. Also, Uranium Oxide (UO2 or U3O8) may be used in place of UNH, if available, and the mass fraction of 235U in the compound (whether UNH or Uranium Oxi...
Disclosures
This work was performed under grant number DE-NA0001983 from the Stewardship Science Academic Alliances (SSAA) Program of the National Nuclear Security Administration (NNSA).
Acknowledgements
Portions of this study have been previously published in the Journal of Radioanalytical and Nuclear Chemistry.3,13 A patent is pending for this method.
Materials
| Name | Company | Catalog Number | Comments |
| High Temperature Furnace (HTF) | Carbolite | HTF 18 | 1,800 °C HTF used to melt samples |
| High Temperature Drop Furnace | CM Inc. | 1706 BL | 1,700 °C Drop Furnace used to melt samples |
| Graphite Crucibles | SCP Science | 040-060-041 | 27 ml high purity graphite crucibles (10 pack) |
| Crucible Tongs | Grainger | 5ZPV0 | 26 in., stainless steele tongs for handling crucibles |
| Heat Resistent Gloves | Grainger | 8814-09 | Gloves used to protect hands from heat during sample intro/removal |
| Mortar & Pestle | Fisherbrand | S337631 | 300 ml, Ceramic mortar and pestle for powdering and mixing |
| Micro Balance | Grainger | 8NJG2 | 220 g Cap, high precision scale for measuring powder mass |
| Spatulas | Fisherbrand | 14374 | Metal spatulas for measure small quantities of powder |
| SiO2 | Sigma-Aldrich | 274739-5KG | Quartz Sand CAS Number: 14808-60-7 |
| Al2O3 | Sigma-Aldrich | 11028-1KG | Aluminum Oxide Powder CAS Number: 1344-28-1 |
| CaO | Sigma-Aldrich | 12047-2.5KG | Calcium Oxide Powder CAS Number: 1305-78-8 |
| FeO | Sigma-Aldrich | 400866-25G | Iron Oxide Powder CAS Number: 1345-25-1 |
| MgO | Sigma-Aldrich | 342793-250G | Magnesium Oxide Powder CAS Number: 1309-48-4 |
| Na2O | Sigma-Aldrich | 36712-25G | Sodium Oxide Powder CAS Number: 1313-59-3 |
| KOH | Sigma-Aldrich | 278904-250G | Potasium Hydroxide Pellets CAS Number: 12030-88-5 |
| MnO | Sigma-Aldrich | 377201-500G | Manganese Oxide Powder CAS Number: 1344-43-0 |
| TiO2 | Sigma-Aldrich | 791326-5G | Titanium Oxide Beads CAS Number: 12188-41-9 |
References
- Carnesdale, A. . Nuclear Forensics: A Capability at Risk (Abbreviated Version). , (2010).
- Garrison, J. R., Hanson, D. E., Hall, H. L. Monte Carlo analysis of thermochromatography as a fast separation method for nuclear forensics. J Radioanal Nucl Chem. 291 (3), 885-894 (2011).
- Molgaard, J. J., et al. Development of synthetic nuclear melt glass for forensic analysis. J Radioanal Nucl Chem. 304 (3), 1293-1301 (2015).
- Fluegel, A. Modeling of Glass Liquidus Temperatures using Disconnected Peak Functions. , (2007).
- Oldham, C. J., Molgaard, J. J., Auxier, J. D., Hall, H. L. Comparison of Nuclear Debris Surrogates Using Powder X-Ray Diffraction. , (2014).
- Liezers, M., Fahey, A. J., Carman, A. J., Eiden, G. C. The formation of trinitite-like surrogate nuclear explosion debris ( SNED ) and extreme thermal fractionation of SRM-612 glass induced by high power CW CO 2 laser irradiation. J Radional Nucl Chem. 304 (2), 705-715 (2015).
- Harvey, S. D., et al. Porous chromatographic materials as substrates for preparing synthetic nuclear explosion debris particles. J Radioanal Nucl Chem. 298 (3), 1885-1898 (2013).
- Hanni, J. B., et al. Liquidus temperature measurements for modeling oxide glass systems relevant to nuclear waste vitrification. J Mater Res. 20 (12), 3346-3357 (2005).
- Weber, W. J., et al. Radiation Effects in Glasses Used for Immobilization of High-Level Waste and Plutonium Disposition. J Mater Res. 12 (8), 1946-1978 (1997).
- Eby, N., Hermes, R., Charnley, N., Smoliga, J. A. Trinitite-the atomic rock. Geol Today. 26 (5), 180-185 (2010).
- Bellucci, J. J., Simonetti, A. Nuclear forensics: searching for nuclear device debris in trinitite-hosted inclusions. J Radioanal Nucl Chem. 293 (1), 313-319 (2012).
- Ross, C. S. . Optical Properties of Glass from Alamogordo, New Mexico. , (1948).
- Giminaro, A. V., et al. Compositional planning for development of synthetic urban nuclear melt glass. J Radional Nucl Chem. , (2015).
- Cook, M. T., Auxier, J. D., Giminaro, A. V., Molgaard, J. J., Knowles, J. R., Hall, H. L. A comparison of gamma spectra from trinitite versus irradiated synthetic nuclear melt glass. J Radioanal Nucl Chem. , (2015).
- Fahey, J., Zeissler, C. J., Newbury, D. E., Davis, J., Lindstrom, R. M. Postdetonation nuclear debris for attribution. Proc Natl Acad Sci U S A. 107 (47), 20207-20212 (2010).
- Bellucci, J. J., Simonetti, A., Koeman, E. C., Wallace, C., Burns, P. C. A detailed geochemical investigation of post-nuclear detonation trinitite glass at high spatial resolution: Delineating anthropogenic vs. natural components. Chem Geol. 365, 69-86 (2014).
- Donohue, P. H., Simonetti, A., Koeman, E. C., Mana, S., Peter, C. Nuclear Forensic Applications Involving High Spatial Resolution Analysis of Trinitite Cross-Sections. J Radioanal Nucl Chem. , (2015).
- Eaton, G. F., Smith, D. K. Aged nuclear explosive melt glass: Radiography and scanning electron microscope analyses documenting radionuclide distribution and glass alteration. J Radioanal Nucl Chem. 248 (3), 543-547 (2001).
- Kersting, A. B., Smith, D. K. . Observations of Nuclear Explosive Melt Glass Textures and Surface Areas. , (2006).
- . . IAEA Safeguards Glossary. , (2001).
- Glasstone, S., Dolan, P. . Effects of Nuclear Weapons. , (1977).
- Carney, K. P., Finck, M. R., McGrath, C. A., Martin, L. R., Lewis, R. R. The development of radioactive glass surrogates for fallout debris. J Radioanal Nucl Chem. 299 (1), 363-372 (2013).
- Molgaard, J. J., Auxier, J. D., Hall, H. L. A Comparison of Activation Products in Different Types of Urban Nuclear Melt Glass. , (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved