Método de adición estándar
Fuente: Laboratorio del Dr. Pablo Bower - Universidad de Purdue
El método de adiciones estándar es un método de análisis cuantitativo, que a menudo se utiliza cuando la muestra de interés tiene varios componentes que resultan en efectos de matriz, donde los componentes adicionales pueden reducir o aumentar la señal de absorbancia del analito. Resulta en errores significativos en los resultados del análisis.
Adiciones estándar se usan para eliminar efectos de matriz de una medición, puesto que se asume que la matriz afecta a todas las soluciones igual. Además, se utiliza para corregir la química fase separaciones realizadas en el proceso de extracción.
El método se realiza mediante la lectura de la intensidad (en este caso fluorescente) experimental de la solución desconocida y luego midiendo la intensidad de lo desconocido con cantidades variables de estándar conocido añadido. Los datos se trazan como fluorescencia intensidad vs. la cantidad del estándar añadido (lo desconocido, con ningún estándar añadido, se traza en el eje y). La línea de mínimos cuadrados se cruza con el eje x en el negativo de la concentración de lo desconocido, como se muestra en la figura 1.

Figura 1. Representación gráfica del método de adición estándar de.
1. preparación de los reactivos
- Al3 + solución estándar de 100 ppm: disolver 0,9151 g de nitrato básico de aluminio (Al (NO3)3•9H2O) en un matraz aforado de 1 L con agua desionizada.
- Solución de 8HQ en 1 M de ácido acético (2% peso/vol): Añadir a un matraz aforado de 100 mL 2,0 g de 8-hidroxiquinoleína.
- Cuidadosamente añadir 5,74 mL ácido acético glacial al matraz de 100 mL y diluir hasta la marca con agua desionizada. Esto permite que la 8-hidro
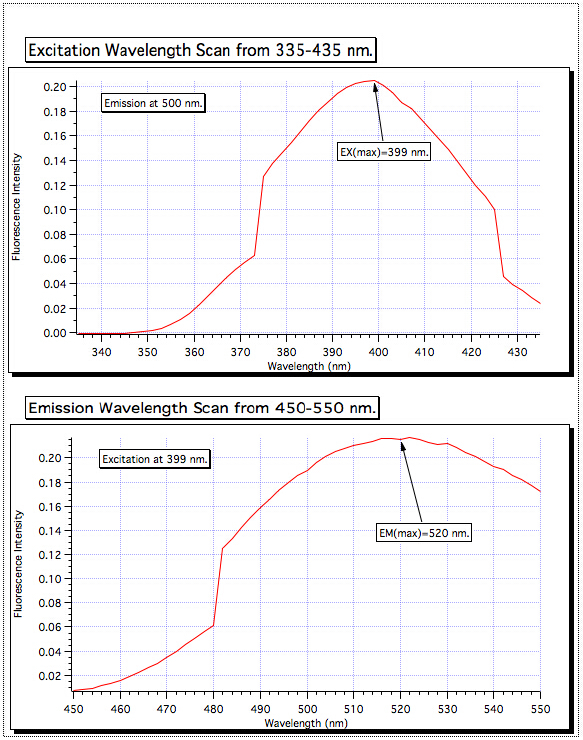
Una exploración de la longitud de onda de excitación de 335-435 demostró la absorción más alta en 399 nm, por lo que el monocromador de excitación fue fijado para ese valor. Luego se realizó la exploración de la emisión de 450-550 nm, y la señal más fuerte fue encontrada para ser a 520 nm. Estas son las longitudes de onda que se utilizan para todas las muestras.