标准加入的方法
Overview
资料来源: 实验室的博士保罗 · 鲍尔-普渡大学
标准加入法是感兴趣的样品已导致基体效应,在附加组件可能减少或增强分析物吸收信号的多个组件时常用的定量分析方法。这导致重大错误的分析结果。
标准附加常用来消除基体效应的测量,因为它假设矩阵同样影响的所有解决方案。此外,它用来纠正这种化学物质的相分离的萃取过程中执行。
通过阅读实验 (在这种情况下荧光) 强度的未知溶液,然后通过测量未知的强度与不同数量的已知标准添加执行该方法。数据绘制为荧光强度 vs.的标准添加量 (未知本身,没有标准补充道,在 y 轴上绘制)。最小二乘行在未知浓度的负 x 轴相交,如图 1所示。

图 1.的标准加入法的图形表示。
Procedure
1.制备试剂
- 100 ppm 标准 Al3 +溶液: 溶解 0.9151 g 硝酸铝 (Al (没有3)3•9H2O) 用去离子水 1 L 容量瓶。
- 1 M 醋酸 (2 %wt / 卷) 的 8HQ 解决方案: 将 2.0 g 8-羟基喹啉添加到 100 毫升的容量瓶。
- 小心地 5.74 毫升冰醋酸加入 100 毫升烧瓶中,然后稀释至马克用去离子水。这允许 8-羟基喹啉,溶解在水相中。
- 1 M NH4+/NH3缓冲区 (pH ~ 8): 将 20 g 的醋酸铵 (NH4OAc) 添加到一瓶 100 毫升。
- 到这瓶 100 毫升,添加 7 毫升 30%氢氧化铵和稀释至马克用去离子水。这有助于中和酸的 8HQ 解决方案结合时中。
- 其他试剂包括无水硫酸钠 (Na2等4) 和氯仿 (规格等级)。
Results
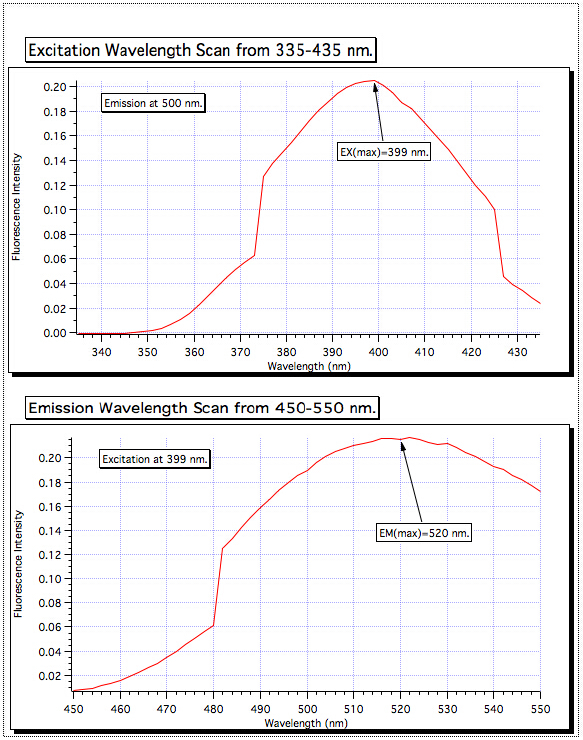
从 335-435 激发波长扫描显示最高吸收在 399 nm,所以励磁单色器被设置为该值。然后从 450-550 nm 进行发射扫描和最强的信号被发现在 520 nm。这些都是用于所有的样品的波长。