Voltametría cíclica (CV)
Visión general
Fuente: Laboratorio del Dr. Kayla Green, Texas Christian University
Un experimento de voltametría cíclica (CV) consiste en la exploración de una gama de posibles tensiones durante la medición actuales. En el experimento de la CV, se explora el potencial de un electrodo sumergido, inmóvil desde un potencial inicial predeterminado en un valor final (llamado la conmutación posible) y luego se obtiene el análisis inverso. Esto le da un 'cíclico' barrido de potenciales y la corriente vs curva derivada de los datos se llama un voltagrama cíclico. El barrido de la primera se llama 'scan forward' y la onda de retorno se llama la 'exploración inversa'. Los extremos de potencial se denominan la 'ventana de exploración'. La magnitud de las corrientes de reducción y oxidación y la forma de los voltamperogramas son muy dependientes de la concentración de analito, exploración de precios y condiciones experimentales. Mediante la variación de estos factores, voltametría cíclica puede producir información sobre la estabilidad del estado de oxidación del metal de la transición en la forma acomplejada, reversibilidad de las reacciones de transferencia de electrones e información sobre la reactividad. Este video explica la configuración básica para un experimento de voltametría cíclica como analito preparación y configuración de la celda electroquímica. Se presentará un experimento simple de voltametría cíclica.
Procedimiento
1. preparación de solución electrolítica
- Prepare una electrólito solución (10 mL) compuesta de 0.1 M [Bu4N] [BF4] en CH3CN.
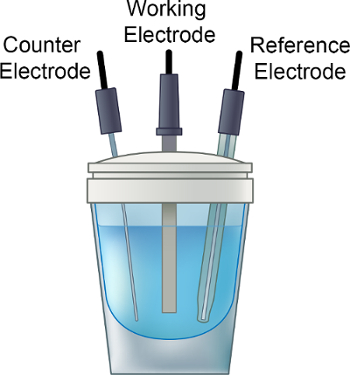
- Coloque la solución electrolítica en el frasco de electroquímico, añadir una barra de agitación pequeño y coloque la tapa sobre el frasco, como se muestra en la figura 1.
- Verifique para asegurarse de que el nitrógeno es en la solución electrolítica. Revuelva y desgasificar la solución electrolítica con una corriente su
Resultados
Se realizó un análisis de la CV de ferroceno en 300 mV/s en acetonitrilo y el voltagrama correspondiente se muestra en la figura 2.
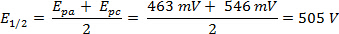
La ΔE puede derivar de los datos en la figura 2 la diferencia entre Epa y Epc.
Log in or to access full content. Learn more about your institution’s access to JoVE content here
Tags
Saltar a...
Vídeos de esta colección:

Now Playing
Voltametría cíclica (CV)
Analytical Chemistry
126.0K Vistas

Preparación de muestras para la caracterización analítica
Analytical Chemistry
85.2K Vistas

Estándares internos
Analytical Chemistry
205.4K Vistas

Método de adición estándar
Analytical Chemistry
320.8K Vistas

Curvas de calibración
Analytical Chemistry
798.7K Vistas

Espectroscopía ultravioleta-visible (UV-Vis)
Analytical Chemistry
625.2K Vistas

Espectroscopía de Raman para el análisis químico
Analytical Chemistry
51.4K Vistas

Fluorescencia de rayos x (XRF)
Analytical Chemistry
25.9K Vistas

Cromatografía de gases (CG) con detección de ionización de llama
Analytical Chemistry
283.1K Vistas

Cromatografía de líquidos de alto rendimiento (HPLC)
Analytical Chemistry
386.0K Vistas

Cromatografía de intercambio iónico
Analytical Chemistry
265.1K Vistas

Electroforesis capilar (EC)
Analytical Chemistry
94.5K Vistas

Introducción a la espectrometría de masas
Analytical Chemistry
112.9K Vistas

Microscopía electrónica de barrido (MEB)
Analytical Chemistry
87.6K Vistas

Mediciones electroquímicas de catalizadores soportados utilizando un potenciostato/galvanostato
Analytical Chemistry
51.8K Vistas
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados