1. du visage veine saigner chez la souris
- Matériel
- Collecte de sang est d’une prise libre dans un tube de sang ou un tube Eppendorf. Dans certains cas, il convient de prélever le sang directement dans les tubes de l’hématocrite.
- Lancettes de verge d’or seront choisis en fonction de la taille appropriée pour l’animal selon l’âge et le sexe.
- Les lancettes sont choisis selon l’âge et la taille des souris comme suit :
lancet 4mm : 3-4-week-old souris (moins de 15 grammes de poids corporel)
lancet 5mm : souris femelles de moins de 10 semaines (moins de 20 grammes de poids corporel)
lancet 5mm : souris mâles de moins de 6 semaines (moins de 20 grammes de poids corporel)
lancet 5mm : pour les échantillons de goutte à goutte (pour les frottis sanguins)
lancet 5,5 mm : souris femelles de plus de 10 semaines (plus environ 20 grammes de poids corporel)
lancet 5,5 mm : souris mâles pendant 6 semaines (plus environ 20 grammes de poids corporel)
lancet 5,5 mm : grands échantillons
- Dispositif de retenue pour
- Souris sont immobilisées à l’aide de la technique scruffing.
- Il est important que le mouvement à côté de la tête être minimisé. Cela garantit une ponction précise et sûre avec la lancette.
- Prélèvement de sang
- La lancette de bonne taille est tenue perpendiculairement à la surface de la peau.
- Le point de la lancet est légèrement incliné, avec la pointe tournée vers le nez.
- La lame de bistouri est mieux utilisée en position verticale.
- Tout en retenant la souris, localisez la superficie approximative de la veine faciale en mesurant la longueur de le œil sous le canthus latéral et la largeur de l’oeil direction caudale.
- Avec la pointe de la lancette, doucement sentir pour le point où se termine la mâchoire.
- Pour une meilleure précision en perforant le navire, positionnez la souris en décubitus latéral.
- Percer la peau de l’épaule de la lancette à ce moment-là. Cela se fait avec une pression ferme et non comme lancer une fléchette.
- Lors du retrait de la lancette, sang commencera à couler.
- Pour aider la circulation sanguine, positionnez la souris avec la tête plus bas que le coeur.
- Recueillir le sang dans le vaisseau de la collection désirée.
- Épongez la pression de site et de la libération de ponction sur la peau du cou pour l’hémostase.
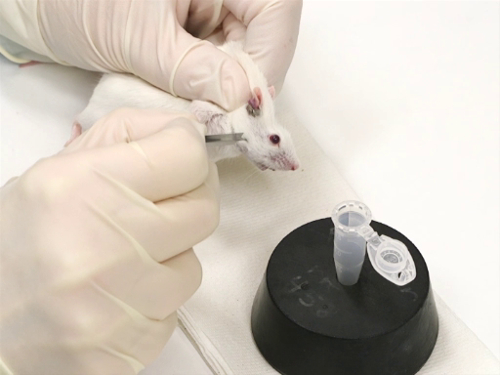
La figure 1. Purge de veine faciale chez les souris.
2. sous-maxillaire saigner chez la souris : bien que très similaire à la technique pour la purge de la veine faciale, il y a des variations dans l’équipement et des différences subtiles dans cette procédure de purge.
- Matériel
- Collecte du sang est d’une prise libre dans un tube de sang ou un tube Eppendorf. Dans certains cas, il est souhaitable de recueillir directement dans des tubes de l’hématocrite.
- aiguilles de calibre 18-22 sont choisis en fonction de la taille appropriée pour l’animal selon l’âge et le sexe.
- Aiguilles sont choisis selon l’âge et la taille des souris comme suit :
calibre 22 : souris vieux de 3 à 4 semaines (moins de 15 grammes de poids corporel)
calibre 20 : souris femelles de moins de 10 semaines (moins de 20 grammes de poids corporel)
calibre 20 : souris mâles de moins de 6 semaines (moins de 20 grammes de poids corporel)
calibre 20 : pour les échantillons de goutte à goutte (pour les frottis sanguins)
calibre 18 : souris femelles de plus de 10 semaines (plus environ 20 grammes de poids corporel)
calibre 18 : souris mâles pendant 6 semaines (plus environ 20 grammes de poids corporel)
calibre 18 : grands échantillons
- Dispositif de retenue pour
- Souris sont immobilisées à l’aide de la technique scruffing.
- Il est important que le mouvement à côté de la tête être minimisé. Cela garantit la ponction veineuse précise et sûre avec l’aiguille.
- Prélèvement de sang
- L’aiguille est tenue perpendiculairement à la surface de la peau.
- Tout en retenant la souris, localisez la zone approximative de la veine sous-maxillaire par le point d’intersection de ligne en partant du coin de la bouche sur une ligne allant du canthus latéral de le œil. Cela coïncide avec une fossette petite, glabre, trouvée caudale à l’angle de la bouche et un peu au-dessous de la ligne de la mâchoire.
- Pour une meilleure précision en perforant le navire, positionnez la souris en décubitus latéral.
- Percer la peau avec la pointe de l’aiguille à ce moment-là. Cela se fait avec une pression ferme et non comme lancer une fléchette.
- L’aiguille n’est pas insérée au-delà de l’extrémité du biseau.
- Lors du retrait de l’aiguille, le sang commencera à couler.
- Pour aider la circulation sanguine, positionnez la souris avec la tête plus bas que le coeur.
- Recueillir le sang dans le vaisseau de la collection désirée.
- Épongez le site de ponction et relâcher la pression sur la peau du cou pour l’hémostase.
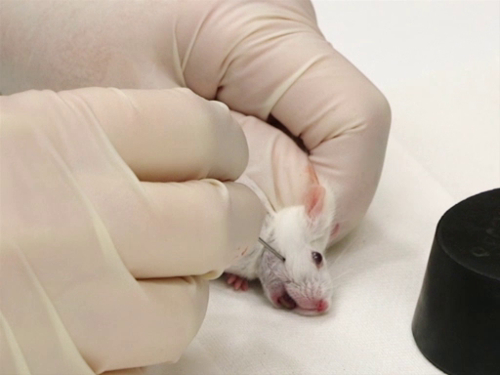
La figure 2. Purge de veine sous-mandibulaire chez la souris.
3. saphène purge
- Matériel
- Cônes en plastique transparent, souple retenue peuvent être utilisés pour la souris ou le rat. Pour les souris, mis à jour le 50 millilitre de tubes coniques en plastique peuvent être utilisés à la retenue. Pour les rats, modifiée tubes retenue en Plexiglas-avec une fente large assez pour étendre la jambe arrière-peut être utilisé.
- Lorsque vous utilisez le cône en plastique, il est mesuré la longueur du corps de l’animal, et un trou ovale est coupé au niveau de la cuisse.
- Un tube conique peut être modifié pour une souris pour cette méthode de collecte de sang en coupant l’extrémité du tube pour permettre un trou de respiration. Une fente est coupée de la fin de la PAC de la tube d’environ ½ pouce de large et 2 pouces de long. Les bords sont couverts de ruban de toile ou lissées pour la sécurité des animaux.
- Un garrot est fabriqué à l’aide d’une seringue cc 3 et une longueur de suture de 2-0. 3
- Onguent antibiotique triple ou gelée de pétrole blanche est utilisée comme une barrière étanche à l’humidité sur la peau.
- Une aiguille de 22 calibre est la taille par défaut pour la ponction veineuse.
- Sang est prélevé directement dans les tubes de l’hématocrite.
- Dispositif de retenue pour
- Cônes en plastique souples
- La souris ou le rat est placé dans le cône de nez en avant.
- L’extrémité du cône est pliée et fermé à l’aide d’une pince-petites notes pour empêcher l’animal de quitter le cône de retenue.
- La patte est légèrement aspirée dans l’ovale ouvrant à l’aine.
- Tube conique pour souris
- La souris est placée dans le nez en tube avant.
- La patte est guidée doucement dans la fente.
- Le doigt du milieu est placé au-dessus de l’extrémité du tube pour empêcher la souris de quitter le tube.
- L’index et le pouce stabilisent la jambe de la souris.
- Tube de retenue de plexiglas pour les rats
- Le rat est placé dans le nez en tube avant.
- La patte est guidée doucement dans la fente.
- L’extrémité du tube est sécurisée afin d’empêcher le rat de sauvegarder et quitter le tube.
- L’index et le pouce stabilisent la jambe de rat.
- Prélèvement de sang
- Le poil est prélevé à la face latérale de la jambe de la pointe du jarret à l’articulation fémoro. Cela peut être fait par la cueillette, rasage ou à l’aide d’une crème dépilatoire.
- Après avoir retiré les cheveux, une petite quantité d’onguent est appliquée et répandue en une couche très mince sur la zone chauve.
- Le Garrot est appliqué comme beaucoup parotidien que possible et serrés.
- Le navire saphène courir à travers la surface extérieure de la jambe depuis le genou jusqu'à la cheville va commencer à remplir et deviendra surélevé et facile à visualiser.
- Directement sur les vaisseaux sanguins, l’aiguille est maintenu perpendiculaire à la surface de la peau. Perforer le navire. Veillez à ne pas introduire l’aiguille profondément dans la jambe, afin d’éviter toute perforation du muscle ou de frapper des os.
- Le sang sera billes vers le haut sur la surface de la jambe pour la collection avec le tube de l’hématocrite.
- Une fois que le sang a été prélevé, desserrer le garrot et appliquer une pression sur la piqûre pour l’hémostase.
- Une fois que le saignement a cessé, retirer l’animal de la retenue et le retourner à la cage.
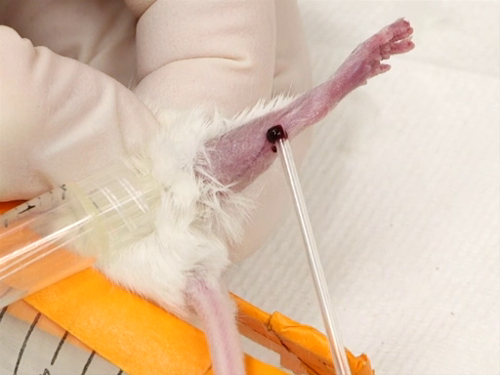
La figure 3. Veine saphène saigner chez la souris.
4. fémorale veine pour les rats
- Matériel
- Le sang est recueilli dans un tube de l’hématocrite.
- Onguent antibiotique triple est nécessaire pour créer une barrière entre la peau et les cheveux et la goutte de sang.
- Une aiguille de 22 calibre est utilisée pour la ponction de la veine.
- Une tondeuse à cheveux petite, portable avec environ une 1" lame large est utilisée pour couper les cheveux de la jambe.
- Dispositif de retenue pour
- Des rats sont retenus à l’aide d’un cône de retenue en plastique clair et flexible.
- Les cônes en plastique sont évalués en fonction de la longueur du corps et un trou ovale coupé au niveau de la cuisse. Le cône est coupé telle que la patte arrière peut être extériorisée avec accès à la veine.
- Prélèvement de sang
- Le poil est rasé de la surface interne de la jambe de l’aine au genou.
- Onguent antibiotique triple s’applique en fine couche sur le site de ponction.
- La personne retenue pour occlusion de la veine et détient le rat avec la surface interne de la jambe vers le prélèvement.
- Utilisez l’aiguille à ponction de la veine. L’aiguille est perpendiculaire au vaisseau sanguin et la ponction se faite directement sur la veine.
- La ponction est faite au plus près du genou que possible, permettant des prélèvements complémentaires avant le premier site de collecte de sang.
- Le navire est superficiel. Par conséquent, la profondeur de la ponction ne doit pas être supérieure à la longueur du biseau de l’aiguille.
- Pour aider la circulation sanguine, placez le rat avec la jambe inférieure à cœur.
- Recueillir le sang dans les tubes d’hématocrite comme il perle sur la surface de la peau.
- Relâcher la pression sur la jambe et appliquer une pression sur le site de ponction pour atteindre l’hémostase.
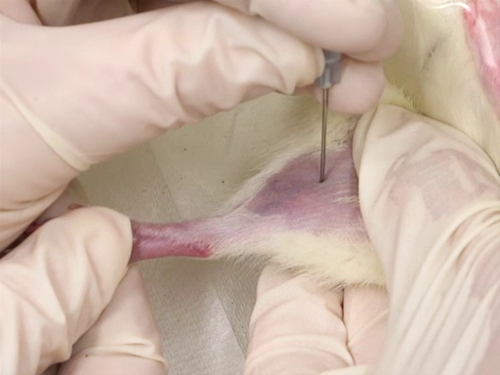
La figure 4. Purge de la veine fémorale chez les rats.


















