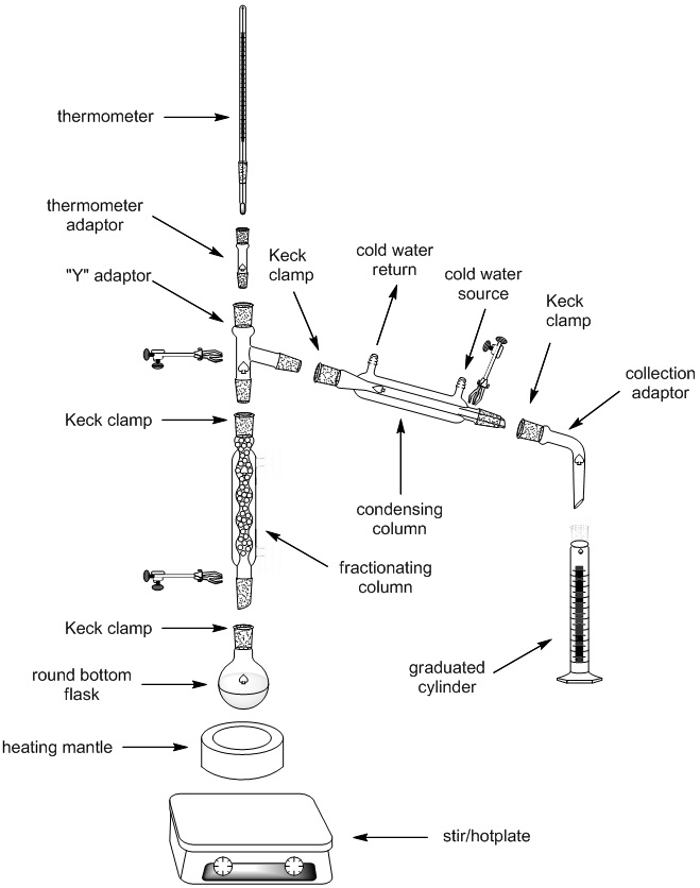
Distillation fractionnée
Vue d'ensemble
Source : Laboratoire de Dr. Nicholas Leadbeater — Université du Connecticut
La distillation est peut-être technique de laboratoire la plus courante utilisée par les chimistes pour l’épuration des liquides organiques. En combinaison avec différents points d’ébullition des composés se divisent en différents composants lorsque le mélange est soigneusement distillé. Les deux principaux types de distillation sont « simple distillation » et « distillation fractionnée », et les deux sont employés couramment dans les laboratoires de chimie organique.
Distillation simple est utilisée lorsque le liquide est b relativement pur (contenant des contaminants liquides de pas plus de 10 %), (b) a une composante non volatile comme un contaminant solide, ou (c) est mélangé avec un autre liquide dont le point d’ébullition qui diffère au moins 25 ° C. Distillation fractionnée est utilisée lorsque la séparation de mélanges de liquides dont les points d’ébullition sont plus semblables (séparés par moins de 25 ° C).
Cette vidéo détaille la distillation fractionnée d’un mélange des deux solvants organiques communs, cyclohexane et le toluène.
Procédure
1. mise en place d’appareils de Distillation fractionnée