Ion-Exchange Chromatography
Source: Laboratory of Dr. B. Jill Venton - University of Virginia
Ion-exchange chromatography is a type of chromatography that separates analytes based on charge. A column is used that is filled with a charged stationary phase on a solid support, called an ion-exchange resin. Strong cation-exchange chromatography preferentially separates out cations by using a negatively-charged resin while strong anion-exchange chromatography preferentially selects out anions by using a positively-charged resin. This type of chromatography is popular for sample preparation, for example in the cleanup of proteins or nucleic acid samples.
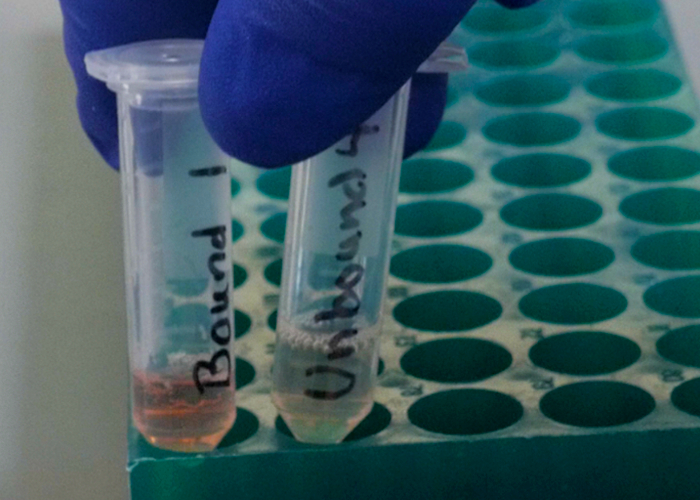
Ion-exchange chromatography is a two-step process. In the first step, the sample is loaded onto the column in a loading buffer. The binding of the charged sample to the column resin is based on ionic interactions of the resin to attract the sample of the opposite charge. Thus, charged samples of opposite polarity to the resin are strongly bound. Other molecules that are not charged or are of the opposite charge are not bound and are washed through the column. The second step is to elute the analyte that is bound to the resin. This is accomplished with a salt gradient, where the amount of salt in the buffer is slowly increased. Fractions are collected at the end of the column as the elution occurs and the purified sample of interest can be recovered in one of these fractions. Another technique, such as spectroscopy, may be needed to identify which fraction contains the sample. Ion-exchange chromatography is especially useful in protein studies, to isolate proteins of interest that have a specific charge or size, as size can determine the number of interactions with the resin.
Ion-exchange chromatography is a more general separation technique than affinity chromatography, which is also often used in preparing protein samples, where an antibody is attached to a column to bind one specific analyte. A new affinity column must be purchased for each analyte, while the same type of ion-exchange column, often with different eluting conditions, can be used to clean up many proteins of the same charge. Ion-exchange chromatography can also be used in conjunction with other types of chromatography that separate based on other properties. For example, size-exclusion chromatography separates based on size and could be used before ion-exchange chromatography to choose compounds of only a given size.
1. Preparing the Sample and the Column
- In this demonstration, a mixture of 2 proteins will be separated on a cation-exchange column: hemoglobin and cytochrome C. Add 0.2 mL equilibration buffer (pH 8.1) to the protein sample and vortex to mix thoroughly. Centrifuge for 2 min to remove any froth.
- Place the cation-exchange column in a test tube for 5 min to allow resin to settle. Clamp the test tube with the column onto a ring stand to make sure it is upright.
- Open the top cap of the column,
Ion-exchange chromatography is widely used in biochemistry to isolate and purify protein samples. Proteins have many amino acids with functional groups that are charged. Proteins are separated based on net charge, which is dependent on pH. Some proteins are more positively charged while others are more negatively charged. In addition, peptide tags can be genetically added to a protein to give it an isoelectric point that is not in the range of normal proteins, making it possible to separate completely. Ion-exchange chrom
Vai a...
Video da questa raccolta:

Now Playing
Ion-Exchange Chromatography
Analytical Chemistry
263.0K Visualizzazioni

Preparazione del campione per la caratterizzazione analitica
Analytical Chemistry
83.7K Visualizzazioni

Standard interni
Analytical Chemistry
203.3K Visualizzazioni

Metodo delle aggiunte standard
Analytical Chemistry
318.8K Visualizzazioni

Curve di calibrazione
Analytical Chemistry
789.0K Visualizzazioni

Spettroscopia ultravioletta/visibile (UV-VIs)
Analytical Chemistry
616.4K Visualizzazioni

Spettroscopia Raman per analisi chimiche
Analytical Chemistry
50.8K Visualizzazioni

Fluorescenza a raggi X (XRF)
Analytical Chemistry
25.3K Visualizzazioni

Gascromatografia con rivelatore a ionizzazione di fiamma
Analytical Chemistry
279.8K Visualizzazioni

Cromatografia liquida ad alta prestazione (HPLC)
Analytical Chemistry
381.8K Visualizzazioni

Elettroforesi capillare
Analytical Chemistry
93.0K Visualizzazioni

Introduzione alla spettrometria di massa
Analytical Chemistry
111.4K Visualizzazioni

Microscopia elettronica a scansione (SEM)
Analytical Chemistry
86.6K Visualizzazioni

Misurazioni elettrochimiche di catalizzatori supportati mediante l'utilizzo di un potenziometro/galvanometro
Analytical Chemistry
51.2K Visualizzazioni

Voltammetria ciclica
Analytical Chemistry
123.4K Visualizzazioni