Assorbitore di gas
Panoramica
Fonte: Michael G. Benton e Kerry M. Dooley,Dipartimento di Ingegneria Chimica, Louisiana State University, Baton Rouge, LA
Gli assorbitori di gas vengono utilizzati per rimuovere i contaminanti dai flussi di gas. Per raggiungere questo obiettivo vengono utilizzati più progetti1. Una colonna a letto imballata utilizza flussi di gas e liquidi che scorrono l'uno contro l'altro in una colonna imballata con materiali di imballaggio sfusi, come ceramica, metalli e plastica, o imballaggi strutturati1. Il letto imballato utilizza la superficie creata dall'imballaggio per creare una quantità massima di contatto efficiente tra le due fasi1. I sistemi sono a bassa manutenzione e possono gestire materiali corrosivi con elevate velocità di trasferimento di massa1. Le colonne di spruzzatura sono un altro tipo di assorbitore, che utilizza un contatto diretto costante tra le due fasi, con il gas che si muove verso l'alto e il liquido che viene spruzzato nel flusso di gas1. Questo sistema ha solo uno stadio e scarse velocità di trasferimento di massa, ma è molto efficace per i soluti con elevata solubilità liquida1.
L'obiettivo di questo esperimento è determinare in che modo le variabili tra cui la portata del gas, la portata d'acqua e la concentrazione di anidride carbonica influenzano il coefficiente di trasferimento di massa complessivo in un assorbitore di gas. Capire come questi parametri influenzano la rimozione di CO2 consente di ottimizzare la rimozione dei contaminanti. L'esperimento utilizza una colonna di assorbimento del gas controcorrodo dell'acqua imballata in modo casuale. Sono state utilizzate otto serie con due diverse portate di gas, portate liquide e concentrazioni di CO2. Durante ogni corsa, le pressioni parziali sono state prelevate dal basso, dal centro e dalla parte superiore dell'unità della colonna e sono state calcolate le pressioni parziali di equilibrio. Queste pressioni sono state poi utilizzate per trovare il coefficiente di trasferimento di massa e i coefficienti di trasferimento di massa sono stati confrontati con i valori teorici.
Principi
Un'unità di assorbimento del gas (Figura 1) utilizza il contatto con un liquido per rimuovere una sostanza da una miscela di gas. La massa viene trasferita dalla miscela di gas al liquido tramite assorbimento.

Figura 1: Tipica colonna di assorbimento del gas.
Il coefficiente di trasferimento di massa complessivo è la velocità con cui la concentrazione di una specie si sposta da un fluido all'altro (Equazione 1).
 (1)
(1)
Nell'equazione 1, Gs è la portata molare del gas per area della sezione trasversale della colonna, pAg è la pressione parziale di CO 2 , p*A è lapressionein equilibrio con pAg, a è l'area / volume interfacciale o "area effettiva" (una funzione dell'imballaggio della colonna), z è l'altezza dell'imballaggio, e KG è il coefficiente di trasferimento di massa complessivo in mols/(pressione x area interfacciale x tempo). Il trasferimento di massa dipende dai coefficienti di trasferimento di massa in ciascuna fase e dalla quantità di area interfacciale disponibile nell'assorbitore. La legge di Henry o legge di Raoult viene applicata per approssimare le pressioni parziali. Sono due leggi che descrivono la pressione parziale di un componente in una miscela e sono usate insieme per descrivere pienamente il comportamento della miscela ai limiti del rapporto di equilibrio vapore-liquido. L'obiettivo di una colonna di assorbimento del gas è controllare la pressione parziale dell'effluente del contaminante. Un solvente liquido fluisce controcorrente al flusso di gas per rimuovere il contaminante attraverso il trasferimento di massa convettivo. Il trasferimento di massa complessivo di una colonna impacchettata in controcorrodo d'acqua viene misurato in questo studio per determinare gli effetti del flusso d'acqua, del flusso di gas e della concentrazione di gas CO2. I coefficienti saranno poi confrontati con i valori teorici.
Procedura
L'esperimento utilizza una colonna di assorbimento del gas controcorrodo dell'acqua imballata in modo casuale. La colonna è imballata con selle da 34 cm di 13 mm con 465 m2/ m3 superficie (effettiva). La pressione che entra nel sistema è di circa 1,42 bar con una temperatura di circa 26 °C, e le valvole all'ingresso e all'uscita della colonna consentono al gas di fuoriuscire. Uno spettrometro a infrarossi "Oxy Baby", collegato direttamente all'unità in varie posizioni, misura la composizione del gas e i serbatoi di gas puro vengono utilizzati per la calibrazione.
1. Funzionamento dell'assorbitore di gas
- Accendere l'interruttore principale e chiudere la valvola di regolazione utilizzata per controllare la quantità di acqua nella colonna
- Aprire completamente la valvola di flusso d'aria e la valvola di regolazione per la pressione della colonna.
- Impostare la portata d'aria al livello desiderato (utilizzare un minimo di 20 L / min e aumentare secondo necessità) e impostare la pressione della colonna a ~ 1,4 bar e 25 ° C utilizzando la valvola di regolazione per la pressione.
- Avviare la portata dell'anidride carbonica a ~ 4 L / min.
- Impostare il flusso d'acqua a ~ 75 L / h e regolare il livello dell'acqua per mantenere un'altezza costante. Modificare se necessario durante la corsa per garantire un'altezza costante.
- Campionare la pressione parziale di CO2 alla base, al centro e alla testa della colonna utilizzando i rubinetti a pressione e lo spettrometro a infrarossi.
- Esegui otto diverse corse, utilizzando due diverse portate di gas, portate di liquidi e concentrazioni di CO2. Ciò consentirà di determinare le variabili più importanti.
- Consentire al sistema di raggiungere lo stato stazionario quando viene alterata una portata. Questo richiede in genere 30 - 45 minuti.
Risultati
Pressioni parziali sono state prese da ogni prova. I coefficienti di trasferimento di massa sono stati calcolati da questi e confrontati con i valori previsti (Figura 2). I valori previsti derivano dalla linea operativa calcolata per l'assorbitore (vedere il riferimento 2 per una discussione approfondita della linea operativa). Le linee continue rappresentano i valori calcolati utilizzando la linea operativa, mentre i triangoli rappresentano i valori sperimentali del coefficiente di trasferimento di massa. Gli intervalli di confidenza per i valori del modello e il coefficiente di trasferimento di massa medio sono stati tracciati con linee tratteggiate. Questi valori sono stati confrontati per determinare in che modo i parametri sperimentali (portata liquida, portata del gas e pressione parziale di CO2) hanno influenzato il coefficiente di trasferimento di massa complessivo. In queste condizioni operative, solo la portata del liquido ha avuto un effetto statisticamente significativo sul trasferimento di massa rispetto all'intervallo di confidenza. I risultati hanno mostrato che la portata del gas e la composizione dell'alimentazione hanno avuto poco o nessun effetto sul coefficiente di trasferimento di massa.

Figura 2: Modello dei valori previsti ed effettivi del coefficiente di trasferimento di massa.
I valori teorici di KG per un alto (30 L/min) e un basso (20 L/min) sono stati calcolati dalle correlazioni dei coefficienti di trasferimento di massa e sono mostrati rispettivamente come linee blu e verdi nella Figura 3. I valori sperimentali di KG a una varietà di portate liquide sono stati tracciati rispetto ai valori teorici e hanno mostrato tendenze simili, verificando la dipendenza di KG dalla portata del liquido. I valori teorici hanno mostrato qualche variazione rispetto ai valori sperimentali, attribuibile a piccoli errori sperimentali.
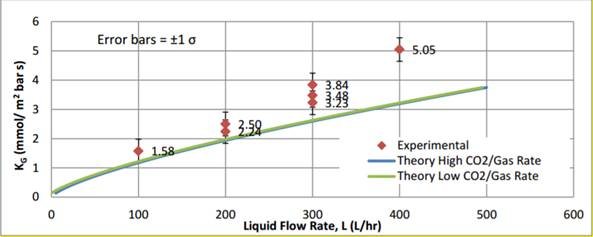
Figura 3: Rappresentazione grafica del valore sperimentale rispetto ai valori teorici.
Applicazione e Riepilogo
L'obiettivo di questo esperimento era quello di utilizzare fattori di portata del gas, portata d'acqua e concentrazione di anidride carbonica per determinare il coefficiente di trasferimento di massa complessivo in un assorbitore di gas. L'esperimento ha utilizzato una colonna di assorbimento del gas controcorrodo dell'acqua GUNT CE 400 imballata in modo casuale. Sono state eseguite otto corse con due diverse portate di gas, portate di liquidi e concentrazioni di CO2. Le pressioni parziali sono state prese dal basso, dal centro e dalla parte superiore dell'unità della colonna, e queste pressioni sono state poi utilizzate per trovare il coefficiente di trasferimento di massa.
In queste condizioni operative, solo la portata del liquido ha avuto un effetto statistico significativo sul trasferimento di massa rispetto all'intervallo di confidenza per le condizioni date. Il processo è controllato dal trasferimento di massa in fase liquida. I fattori correlati al gas come la concentrazione di CO2 e la portata del gas avranno poco o nessun significato.
L'assorbimento di gas è un meccanismo importante per la sicurezza nella produzione di cloro3. Durante il normale funzionamento, gli assorbitori di gas trattano eventuali perdite che si verificano costantemente. L'avvio di un'operazione di cloro deve essere trattato fino a quando non produce un prodotto privo di gas. In caso di guasto del processo, gli assorbitori devono essere utilizzati per trattare il gas prodotto. Inoltre, quando si formano nuove perdite, l'unità di risposta alle emergenze principale è l'assorbitore di gas di standby. Le unità di trattamento sono di vitale importanza in queste condizioni operative, in quanto contribuiscono a creare un ambiente sicuro quando si tratta di un prodotto pericoloso3.
Durante la raffinazione del gas naturale, le torri di assorbimento vengono utilizzate per rimuovere i liquidi di gas naturale dalla fase di gas4. Un olio assorbente con affinità con i liquidi del gas naturale rimuove il liquido dalla fase gassosa, purificando il prodotto. L'olio con liquidi di gas naturale viene poi ulteriormente purificato per recuperare i liquidi, come butano, pentani e altre molecole. L'olio può quindi essere riutilizzato per il trattamento.
L'assorbimento viene anche utilizzato per rimuovere le principali impurità CO2 e H2S dal gas naturale della testa di pozzo, convertendolo in gas di gasdotto. Il processo utilizza ammine acquose o glicoli come solventi a basse temperature (tipicamente <40 °C)5.
Tags
Vai a...
Video da questa raccolta:

Now Playing
Assorbitore di gas
Chemical Engineering
36.9K Visualizzazioni

Verifica dell'efficienza del trasferimento di calore di uno scambiatore di calore a tubi alettati
Chemical Engineering
18.0K Visualizzazioni

Utilizzo di un essiccatore a vassoio per studiare il trasferimento di calore convettivo e conduttivo
Chemical Engineering
44.0K Visualizzazioni

Viscosità delle soluzioni di glicole propilenico
Chemical Engineering
33.2K Visualizzazioni

Porosimetria della polvere di silicato di alluminio
Chemical Engineering
9.7K Visualizzazioni

Dimostrazione del modello Power Law per estrusione
Chemical Engineering
10.3K Visualizzazioni

Equilibrio vapore-liquido
Chemical Engineering
89.6K Visualizzazioni

L'effetto del rapporto di riflusso sull'efficienza della distillazione dei vassoi
Chemical Engineering
77.9K Visualizzazioni

Efficienza di estrazione liquido-liquido
Chemical Engineering
48.6K Visualizzazioni

Reattore in fase liquida: inversione del saccarosio
Chemical Engineering
9.7K Visualizzazioni

Cristallizzazione dell'acido salicilico mediante modificazione chimica
Chemical Engineering
24.3K Visualizzazioni

Flusso monofase e bifase in un reattore a letto impaccato
Chemical Engineering
19.0K Visualizzazioni

Cinetica di polimerizzazione per addizione al polidimetilsilossano
Chemical Engineering
16.4K Visualizzazioni

Reattore catalitico: Idrogenazione dell'etilene
Chemical Engineering
30.5K Visualizzazioni

Valutazione del trasferimento di calore di uno Spin-and-Chill
Chemical Engineering
7.4K Visualizzazioni