このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Method Article
傾斜孔サイズを有するポリ(乳酸)スキャフォールドを製造するために簡便かつ環境に優しいルート
要約
In this work, poly(lactic acid)/polyethylene glycol (PLA/PEG) scaffolds were prepared by using a combination of melt mixing and selective leaching. The method herein discussed permitted to develop three-layer scaffolds by highly controlling both porosity and pore size. The mechanical properties were also evaluated in a physiological environment.
要約
Over the recent years, functionally graded scaffolds (FGS) gaineda crucial role for manufacturing of devices for tissue engineering. The importance of this new field of biomaterials research is due to the necessity to develop implants capable of mimicking the complex functionality of the various tissues, including a continuous change from one structure or composition to another. In this latter context, one topic of main interest concerns the design of appropriate scaffolds for bone-cartilage interface tissue. In this study, three-layered scaffolds with graded pore size were achieved by melt mixing poly(lactic acid) (PLA), sodium chloride (NaCl) and polyethylene glycol (PEG). Pore size distributions were controlled by NaCl granulometry and PEG solvation. Scaffolds were characterized from a morphological and mechanical point of view. A correlation between the preparation method, the pore architecture and compressive mechanical behavior was found. The interface adhesion strength was quantitatively evaluated by using a custom-designed interfacial strength test. Furthermore, in order to imitate the human physiology, mechanical tests were also performed in phosphate buffered saline (PBS) solution at 37 °C. The method herein presented provides a high control of porosity, pore size distribution and mechanical performance, thus offering the possibility to fabricate three-layered scaffolds with tailored properties by following a simple and eco-friendly route.
概要
The interest in biodegradable polymers has grown in importance during the last years both in academia and in the industry, due to the rising concerns regarding plastic waste and the reduction in using non-renewable sources1-7. In particular, biocompatible and biodegradable synthetic polymers are widespread in several biomedical application fields, such as drug controlled release8,9, absorbable suture threads8,10, bioprocess intensification11 and tissue engineering12.
Tissue engineering focuses on the development of devices capable to restore and maintain normal function in diseased or injured tissues. Most of the native tissues are composed by different types of cells and extracellular matrices (ECMs) in specific spatial hierarchies. For example, articular cartilage (AC) consists of different zones with varying types and orientations of collagen fibers and collagen-binding proteins. Moreover, cartilage and bone show significantly different hierarchical structures. In this context, the preparation of multilayer scaffolds with engineered properties in each layer could allow replacing heterogeneous tissues by taking into accounts all the local microenvironments of these complex systems13,14.
Based on the cellular/biological and/or physical-chemical characteristics of the scaffolds, the main strategies adopted by the tissue engineering can be divided into monophasic, biphasic, and triphasic. Biphasic and triphasic approaches (BTA) use two or three different pores architectures, materials, or fillers to prepare multilayered functional devices. Furthermore, a single material can be used to achieve biphasic or triphasic devices, as long as it is possible to create a gradient in its physical properties12.
Cell migration plays a key-role in the morphogenesis, inflammation, wound healing and tumor metastasis. Cell movement is encouraged by the presence of a gradient of chemical-physical properties from the surface to the core of the device. Therefore, biomaterials fulfilling the above discussed requirements can be helpful in studying cell migration. In addition to chemical gradients that trigger cells migration (chemotaxis), mechanical properties of cells culture substrate can also lead to cell movement (mechanotaxis)15.
The multilayer structure can also provide the tunable release of specific drugs incorporated within the polymer matrix, by changing the specific area of the layer or the amount of loaded drugs.
Over the past decade, in order to develop scaffolds possessing a discrete or continuous gradient of morphological properties, such as porosity or pore size, several approaches have been presented16-31. The most recent papers focused on the preparation of BTA by adopting: particle leaching 12,28,32, gas foaming technique16, electrospinning17-19, layer by layer casting technique20, rapid prototyping21,22, thermally induced phase separation (TIPS)22, centrifugation freeze drying24,25, triply periodic minimal surfaces (TPMS)26, freeze casting27-30.
Within the frame of this work, we present a fast and simple route to achieve PLA-based three-layer porous scaffolds (TLS), by combining melt mixing, compression molding and salt leaching. Differently from most of the technologies commonly used for scaffold production, the strategy herein adopted can be considered fully eco-friendly, since it does not require any toxic solvent potentially dangerous for environment and for living cells and tissues32. The basic processing-structure-property relationships established in this study by analyzing both morphological features and mechanical behavior of fabricated devices provide guidance to future advances in designing multifunctional graded scaffolds with specific target properties.
プロトコル
1.足場製作
- 20分間実験室ブレンダー中のNaClを粉砕し、100℃のヒーターでそれを乾燥させます。
- 共振で発生することなく、利用可能な最高周波数で30分間ふるい機で乾燥させたNaClを(一度に45グラム)を入れてください。 500ミクロン〜1000ミクロン(M 500)に至るまで、6塩画分を収集します。 300ミクロンから500ミクロン(M 300)へ。 100μmから200μmの(M 100)。 90ミクロンから100(M 90)へ。 図1に図式のような塩の粒子と45ミクロン〜65ミクロン(M 45)から、最終的にM 10は、45ミクロンよりも小さいサイズ。
- 真空は、処理中に加水分解切断を回避するために、一晩、すべての材料を乾燥させます。ガラス転移 - ポリマーの場合に - 各材料については、克服することなく、乾燥の程度を最大化するために温度を選択します。したがって、PEGのためのPLAのためのT = 90℃、T = 25°C、T = NaClを105℃を選択します。
- フィード通常、約後、次にそれぞれ20/5/75の重量パーセント組成を有するPLA、PEGおよびNaCl、T = 190°Cで動作するバッチミキサーおよびN = 60回転数とトルクの一定値を達成するまで、それらを処理し、 10分。その後、急速に得られた材料を集めます。
- 10ミリメートルの直径と3ミリメートルの高さと適切な円筒状の金型でブレンドを入れて、周囲圧力で60秒、3分の180バーでのためにそれらを保つため、210℃で動作する実験用プレスを用いて、単層を準備します。その後、180バールの圧力を維持し、室温でブレンドを冷却します。
- 圧縮成形を介した三層の組立
- 10mmの直径および1mmの高さを有する同一(1.5)に記載されているような方法が異なる金型を用いて、すなわち、各単層を調製します。最後に、6つのディスクは、6つの異なる粒子サイズを含む、10mmの直径および1mmの高さを有し得る:M 500、M 300、M 100、
- フォーR 3層足場A(TLS A)を組み立て、M 500、M 300を積み上げるとM 100、円筒状金型内および周囲圧力で60秒間、210℃で動作する実験用プレスでそれらを圧縮成形し、3分でその後、180バーとは、180バールの圧力を維持し、室温で冷却しました。
注:相互M 90上に積層してTSL Bを準備M 45とM 10と同じ金型及びTLS Aに用いたのと同じ手順に従って、圧縮成形作業を行います
- 円筒型からディスクを削除し、攪拌せずに、沸騰脱塩水浴に入れます。 3時間後、浴から得られた多孔質構造体を除去し、化学フード中、室温で12時間のためにそれらが乾燥してみましょう。
2.形態素解析
- 走査型電子顕微鏡により、足場の形態を評価します。
- 取り付け後、液体窒素下で試料を分解し、接着剤カーボンテープを使用したアルミニウムスタブ上のサンプル。最後に、スパッタcoateを金で撮影前にアルゴン雰囲気下で90秒間の試験中に静電放電を回避するために。
3.足場孔径
- 足場の孔径分布を認識することができる画像処理ソフトウェアを用いてSEM分析によって得られた画像を精巧。
注:この研究では、細孔径分布分析は、MATLABベースのソフトウェアを用いて行った以前33に記載
4.気孔率
- 浸出の前にサンプルを秤量し、次式により理論的な多孔性を評価します:
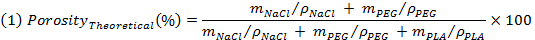
注:NaClを 、 メートルPEGおよびm PLAを M、均質なブレンドを仮定することにより、それぞれのNaCl、PEG、およびPLAの理論上の質量です。 T彼は(ρ)のNaCl、PEG及びPLAのは、それぞれ2,16グラム/ cm 3であり、1,12グラム/ cm 3の電子1,24グラム/ cm 3である密度。 - 試料(ρ 骨格 )の見掛け密度を評価するために浸出し、乾燥後の試料を秤量し、次に足場の見掛け密度との比とすることにより、非多孔質PLAの濃度の逆数として実際の多孔性を評価します式(2)を使用。
注:これは、足場の空のボリュームと(空+フル)足場のフルボリュームとの間の比率を表しています。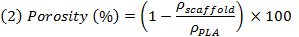
5.機械的性質
- 1 kNのロードセルを装備した引張機を使用して、圧縮モードでサンプルをテストします。分-1 1mmの一定の歪み速度を設定します。
- 生理学的環境におけるサンプルの機械的性能を調べるために、dynamometeを装備37℃で含有する浴(PBS)、(でpH = 7.4)とrと点5.1で説明したものと同じ設定でテストを行います。
- 湿潤環境内の各測定の前に、PBSは、全ての孔を埋めるようにするために5分間の真空フラスコ中で、PBSでサンプルを浸します。その後、足場は設定点温度に到達するために15分間37℃でPBS中に残存することを可能にします。
- 文献32,34に記載の方法以下の機械的試験機に接続されたカスタムデザインの界面強度試験装置を用いてTL AとTL Bの層の間の界面接着強度(IAS)を決定します。
- リグ上の足場を修正して、マシンのロードセルとベースプラテンとの正確な位置合わせを確保します。高粘度の接着剤を用いてアルミニウム試験スタブに足場サンプルを取り付け、テストのための機器に入れます。
- 湿潤状態の試験では、1時間のpri PBS中のサンプルを水和または試験へ。分-1 1mmのひずみ速度で適用される引張荷重の下で、1 kNのロードセルを使用してください。
注:障害がいずれかの層の一つの極限引張強さでため、または界面での剥離の発生する可能性があることを考慮してください。応力 - ひずみ曲線の最大強度としてIASを評価します。
結果
足場の孔のアーキテクチャ上のNaCl粒子サイズの影響は、それぞれ、試料の形態を調査し、画像解析により細孔サイズ分布を計算することによって定性的および定量的に評価した図2a - 。fは 、得られた単層足場のSEM顕微鏡写真を示します別のNaCl粒子サイズを含む材料の塩浸出から。
より詳細には?...
ディスカッション
最初の重要なステップは、効率を篩い分けの最適化です。 NaClの粒径の高い制御は、所望の細孔サイズ分布を有する足場を調製するための基本です。もう一つの重要なステップは、金型からサンプル抽出の際に薄いPLA単層の破壊を回避されています。画像処理解析は、装置全体の代表ではないかもしれません。
引張試験中、サンプルは機器から離れて引き裂くことができ...
開示事項
The authors declare that they have no competing financial interests.
謝辞
This work was financially supported by INSTM.
資料
| Name | Company | Catalog Number | Comments |
| Poly(lactic acid) | NatureWorks | PLA 2002D | |
| Poly(ethylene glycol) | Sigma | 83797-1KG-F | |
| Sodium Cloride | Sigma | 793566-5KG-D | |
| Phosfate Buffer Solution | Sigma | P5368-10PAK | |
| Laboratory Mixer | Brabender | PLE 330 - Plasticorder | |
| Laboratory Press | Carver | ||
| Scanning Electron Microscopy | Phenom-world | ProX | |
| Universal Testing Machine | Instron | 3365 (UK) | |
| BioPuls Bath | Instron, Norwood | ||
| Sieving Machine | Endecotts | E.V.F.1. | |
| Vacuum Oven | ISCO | NSV9035 | |
| Precision Balance | Sartorius | AX224 |
参考文献
- Scaffaro, R., Morreale, M., Lo Re, G., Mantia, F. P. La Degradation of Mater-Bi®/wood flour biocomposites in active sewage sludge. Polymer Degradation and Stability. 94 (8), 1220-1229 (2009).
- Scaffaro, R., Botta, L., Passaglia, E., Oberhauser, W., Frediani, M., Di Landro, L. Comparison of different processing methods to prepare poly(lactid acid)-hydrotalcite composites. Polymer Engineering & Science. 54 (8), 1804-1810 (2014).
- Thakur, V. K., Thakur, M. K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustainable Chemistry and Engineering. 2 (12), 2637-2652 (2014).
- Thakur, V. K., Thakur, M. K. Recent advances in green hydrogels from lignin: A review. International Journal of Biological Macromolecules. 72, 834-847 (2015).
- Thakur, V. K., Kessler, M. R. Self-healing polymer nanocomposite materials: A review. Polymer. 69, 369-383 (2015).
- Thakur, V. K., Thakur, M. K. Recent trends in hydrogels based on psyllium polysaccharide: a review. Journal of Cleaner Production. 82, 1-15 (2014).
- Voicu, S. I., Condruz, R. M., et al. Sericin Covalent Immobilization onto Cellulose Acetate Membrane for Biomedical Applications. ACS Sustainable Chemistry & Engineering. 4 (3), 1765-1774 (2016).
- Scaffaro, R., Botta, L., Sanfilippo, M., Gallo, G., Palazzolo, G., Puglia, A. M. Combining in the melt physical and biological properties of poly(caprolactone) and chlorhexidine to obtain antimicrobial surgical monofilaments. Applied Microbiology and Biotechnology. 97 (1), 99-109 (2013).
- Scaffaro, R., Maio, A., et al. Synthesis and self-assembly of a PEGylated-graphene aerogel. Composites Science and Technology. 128, 193-200 (2016).
- Scaffaro, R., Botta, L., Gallo, G., Puglia, A. M. Influence of Drawing on the Antimicrobial and Physical Properties of Chlorhexidine-Compounded Poly(caprolactone) Monofilaments. Macromolecular Materials and Engineering. 12 (300), 1268-1277 (2015).
- Scaffaro, R., Lopresti, F., et al. Effect of PCL/PEG-Based Membranes on Actinorhodin Production in Streptomyces coelicolor Cultivations. Macromolecular bioscience. 16 (5), 686-693 (2016).
- Scaffaro, R., Lopresti, F., Botta, L., Rigogliuso, S., Ghersi, G. Melt Processed PCL/PEG Scaffold With Discrete Pore Size Gradient for Selective Cellular Infiltration. Macromolecular Materials and Engineering. 301 (2), 182-190 (2016).
- Yousefi, A. -. M., Hoque, M. E., Prasad, R. G. S. V., Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. Journal of Biomedical Materials Research Part A. 103 (7), 2460-2481 (2015).
- Gillette, B. M., Rossen, N. S., et al. Engineering extracellular matrix structure in 3D multiphase tissues. Biomaterials. 32 (32), 8067-8076 (2011).
- Seidi, A., Ramalingam, M., Elloumi-Hannachi, I., Ostrovidov, S., Khademhosseini, A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomaterialia. 7 (4), 1441-1451 (2011).
- Son, J. S., Kim, S. G., et al. Hydroxyapatite/polylactide biphasic combination scaffold loaded with dexamethasone for bone regeneration. Journal of Biomedical Materials Research - Part A. 99 (4), 638-647 (2011).
- Sundararaghavan, H. G., Burdick, J. A. Gradients with depth in electrospun fibrous scaffolds for directed cell behavior. Biomacromolecules. 12 (6), 2344-2350 (2011).
- Zou, B., Liu, Y., Luo, X., Chen, F., Guo, X., Li, X. Electrospun fibrous scaffolds with continuous gradations in mineral contents and biological cues for manipulating cellular behaviors. Acta biomaterialia. 8 (4), 1576-1585 (2012).
- Nedjari, S., Schlatter, G., Hébraud, A. Thick electrospun honeycomb scaffolds with controlled pore size. Materials Letters. 142, 180-183 (2015).
- Yusong, P., Qianqian, S., Chengling, P., Jing, W. Prediction of mechanical properties of multilayer gradient hydroxyapatite reinforced poly(vinyl alcohol) gel biomaterial. Journal of Biomedical Materials Research - Part B Applied Biomaterials. 101 (5), 729-735 (2013).
- Kim, Y. B., Kim, G. Functionally graded PCL/β-TCP biocomposites in a multilayered structure for bone tissue regeneration. Applied Physics A: Materials Science and Processing. 108 (4), 949-959 (2012).
- Sudarmadji, N., Tan, J. Y., Leong, K. F., Chua, C. K., Loh, Y. T. Investigation of the mechanical properties and porosity relationships in selective laser-sintered polyhedral for functionally graded scaffolds. Acta biomaterialia. 7 (2), 530-537 (2011).
- Molladavoodi, S., Gorbet, M., Medley, J., Kwon, H. J. Investigation of microstructure, mechanical properties and cellular viability of poly(L-lactic acid) tissue engineering scaffolds prepared by different thermally induced phase separation protocols. Journal of the mechanical behavior of biomedical materials. 17, 186-197 (2013).
- Oh, S. H., Kim, T. H., Il Im, G., Lee, J. H. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold. Biomacromolecules. 11 (8), 1948-1955 (2010).
- Lin, L., Gao, H., Dong, Y. Bone regeneration using a freeze-dried 3D gradient-structured scaffold incorporating OIC-A006-loaded PLGA microspheres based on β-TCP/PLGA. Journal of Materials Science: Materials in Medicine. 26 (1), 3 (2015).
- Yoo, D. Heterogeneous minimal surface porous scaffold design using the distance field and radial basis functions. Medical engineering & physics. 34 (5), 625-639 (2012).
- Soon, Y. -. M., Shin, K. -. H., Koh, Y. -. H., Lee, J. -. H., Choi, W. -. Y., Kim, H. -. E. Fabrication and compressive strength of porous hydroxyapatite scaffolds with a functionally graded core/shell structure. Journal of the European Ceramic Society. 31 (1-2), 13-18 (2011).
- Scaffaro, R., Lopresti, F., Botta, L., Maio, A. Mechanical behavior of Polylactic acid/Polycaprolactone porous layered functional composites. Composites Part B: Engineering. 98, 70-77 (2016).
- Halili, A. N., Hasirci, N., Hasirci, V. A multilayer tissue engineered meniscus substitute. Journal of Materials Science: Materials in Medicine. 25 (4), 1195-1209 (2014).
- Bai, H., Wang, D., et al. Biomimetic gradient scaffold from ice-templating for self-seeding of cells with capillary effect. Acta Biomaterialia. 20, 113-119 (2015).
- Algul, D., Sipahi, H., Aydin, A., Kelleci, F., Ozdatli, S., Yener, F. G. Biocompatibility of biomimetic multilayered alginate-chitosan/β-TCP scaffold for osteochondral tissue. International Journal of Biological Macromolecules. 79, 363-369 (2015).
- Scaffaro, R., Lopresti, F., Botta, L., Rigogliuso, S., Ghersi, G. Preparation of three-layered porous PLA/PEG scaffold relationship between morphology , mechanical behavior and cell permeability. Journal of the Mechanical Behavior of Biomedical Materials. 54, 8-20 (2016).
- Lo Re, G., Lopresti, F., Petrucci, G., Scaffaro, R. A facile method to determine pore size distribution in porous scaffold by using image processing. Micron. 76, 37-45 (2015).
- Levingstone, T. J., Matsiko, A., Dickson, G. R., O'Brien, F. J., Gleeson, J. P. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomaterialia. 10 (5), 1996-2004 (2014).
- Scaffaro, R., Botta, L., Maio, A., Mistretta, M. C., La Mantia, F. P. Effect of Graphene Nanoplatelets on the Physical and Antimicrobial Properties of Biopolymer-Based Nanocomposites. Materials. 9 (5), 351 (2016).
- Maio, A., Fucarino, R., Khatibi, R., Rosselli, S., Bruno, M., Scaffaro, R. A novel approach to prevent graphene oxide re-aggregation during the melt compounding with polymers. Composites Science and Technology. 119, 131-137 (2015).
- Maio, A., Agnello, S., et al. A rapid and eco-friendly route to synthesize graphene-doped silica nanohybrids. Journal of Alloys and Compounds. 664, 428-438 (2015).
- Maio, A., Giallombardo, D., Scaffaro, R., Piccionello, A. P., Pibiri, I. Synthesis of a fluorinated graphene oxide-silica nanohybrid: improving oxygen affinity. RSC Advances. 6 (52), 46037-46047 (2016).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved