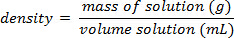水溶液の質量パーセントの組成を決定します。
概要
ソース: 博士ニール エイブラムスの研究室-環境科学および林業のニューヨーク州立大学
溶液の組成を決定する、重要な分析や科学捜査テクニックです。ソリューションは、水で作られています、水溶液、または含んでいる水であると呼ばれます。ソリューションの主なコンポーネントは、溶媒と呼ばれます、溶存微量成分溶質と呼びます。ソリューションを提案する溶剤で溶質を溶解します。水は、ほぼすべての生物学的システムと同様、日常生活の中で最も一般的な溶剤です。化学実験室に溶媒別液、アセトン、エーテル、アルコールなどがあります。溶質は液体または固体で、することができますが、この実験で固体のアドレス決定のみ。
手順
1. 質量 - 直通 %
- クリーンとオーブン乾燥ビーカーまたは結晶皿にソリューションの少量を配置します。
- ソリューションの正確な総質量を正確に判断するには後、ビーカーまたはホット プレート、水をオフ駆動するオーブン皿を加熱します。ゆっくりと蒸発させる最良の方法は、ソリューションの飛散は、沸騰することができます。
- 溶媒が蒸発すると、一度 (溶質) の残りの固体を冷却し、質量を決定します。
- 質量百分率を計算します。
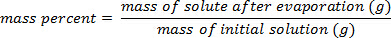
2. 質量の校正曲線を用いた %
- 溶媒に溶質の既知量を溶解することにより標準溶液のシリーズを作る。5 つの規格が推奨され、最大の期待されるパーセント組成範囲最小でなければなりません。
- おおよその値が既知の場合は、0% から水中の溶質の溶解度最大までソリューションのシリーズを生成します。参照テーブ
結果
図 1に示す例を使用して、一連の塩化ナトリウム標準溶液中の溶質の 25.00%、20.00% 15.00% 10.00% 5.000% の質量パーセント組成と用意されていた。測定密度は 1.090 g/mL、1.070、1.060 1.042 1.025 であった。方程式を当てはめて、線形近似曲線が適用されるこれらのデータをプロットした後y = 3.446 x 10-3x + 1.0048, yは、密度、 xは質量パーセント組成。
次に、?...
申請書と概要
ソーダ、砂糖の割合は、determinedusing 質量パーセント組成の原理を簡単にできます。この実験を行うための手順は、質量と脱ソーダ (気泡なし) の量を測定してソリューションの密度を計算するでしょう。対でいくつかの標準的なショ糖 (砂糖) solutionswould 質量パーセント濃度の検量線を作成する必要があり、その校正はソーダでショ糖の割合を解決する使用できます。1 つの仮定は、ショ糖が true 非無糖ソー?...
スキップ先...
このコレクションのビデオ:

Now Playing
水溶液の質量パーセントの組成を決定します。
General Chemistry
383.3K 閲覧数

共通の実験室ガラス製品と用途
General Chemistry
655.3K 閲覧数

・濃度
General Chemistry
273.7K 閲覧数

固体と液体の密度を決定します。
General Chemistry
555.6K 閲覧数

経験式を決定します。
General Chemistry
180.7K 閲覧数

イオン性化合物の溶解度ルールの決定
General Chemistry
141.3K 閲覧数

PH メーターを使用してください。
General Chemistry
345.1K 閲覧数

滴定の概要
General Chemistry
423.9K 閲覧数

理想気体法律
General Chemistry
78.3K 閲覧数

平衡定数の吸光光度定量
General Chemistry
158.3K 閲覧数

ル Châtelier の原理
General Chemistry
264.9K 閲覧数

未知の化合物を決定するための凝固点降下
General Chemistry
160.6K 閲覧数

率の法律および反作用の順序を決定します。
General Chemistry
195.9K 閲覧数

エンタルピーの示差走査熱量測定の変更を使用してください。
General Chemistry
44.4K 閲覧数

錯体化学
General Chemistry
91.3K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved