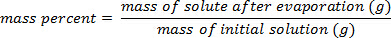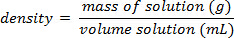Determinazione della composizione percentuale di massa in una soluzione acquosa
Panoramica
Fonte: Laboratorio del Dr. Neal Abrams - SUNY College of Environmental Science and Forestry
Determinare la composizione di una soluzione è un'importante tecnica analitica e forense. Quando le soluzioni sono fatte con acqua, si parla di acqua o contenenti acqua. Il componente primario di una soluzione è indicato come solvente e il componente minore disciolto è chiamato soluto. Il soluto viene sciolto nel solvente per creare una soluzione. L'acqua è il solvente più comune nella vita di tutti i giorni, così come quasi tutti i sistemi biologici. Nei laboratori di chimica, il solvente può essere un altro liquido, come acetone, etere o alcol. Il soluto può essere un liquido o un solido, ma questo esperimento affronta solo la determinazione dei solidi.
Procedura
1. Percentuale di massa - Diretto
- Posizionare un piccolo volume di una soluzione in un becher pulito e essiccato al forno o in una cristallizzazione.
- Dopo aver determinato con precisione la massa totale precisa della soluzione, riscaldare il becher o il piatto su una piastra elettrica o in un forno per scacciare l'acqua. L'evaporazione lenta è il metodo migliore, poiché l'ebollizione può causare schizzi della soluzione.
- Una volta che il solvente è evaporato, raffreddare il solido rimanente (
Risultati
Utilizzando l'esempio mostrato nella Figura 1,è stata preparata una serie di standard di cloruro di sodio con composizioni percentuali di massa del 5.000%, 10.00%, 15.00%, 20.00% e 25.00% di soluto in soluzione. Le densità misurate erano rispettivamente 1,025, 1,042, 1,060, 1,070 e 1,090 g/ml. Dopo aver tracciato questi dati, viene applicata una linea di tendenza lineare, adattandosi all'equazione y = 3,446 x 10-3x + 1,0048, dove y è la densità e x è la co...
Applicazione e Riepilogo
La percentuale di zucchero nella soda, potrebbe essere facilmente determinatausando il principio della composizione percentuale di massa. La procedura per fare questo esperimento sarebbe quella di misurare la massa e il volume della soda degassata (senza bolle) e calcolare la densità della soluzione. Sarebbe necessario creare una curva di calibrazione della densità rispetto alla percentuale in massa per diverse soluzioni standard di saccarosio (zucchero), e quindi tale calibrazione potrebbe essere utilizzata per risolvere la percentuale di..
Vai a...
Video da questa raccolta:

Now Playing
Determinazione della composizione percentuale di massa in una soluzione acquosa
General Chemistry
383.9K Visualizzazioni

Vetreria di laboratorio e relativi usi
General Chemistry
659.6K Visualizzazioni

Soluzioni e concentrazioni
General Chemistry
275.6K Visualizzazioni

Determinazione della densità di un solido e di un liquido
General Chemistry
557.2K Visualizzazioni

Determinazione della formula empirica (minima)
General Chemistry
183.9K Visualizzazioni

Determinazione delle regole di solubilità di composti ionici
General Chemistry
141.6K Visualizzazioni

Uso del pH-metro
General Chemistry
347.1K Visualizzazioni

Introduzione alla titolazione
General Chemistry
425.8K Visualizzazioni

Legge dei gas perfetti
General Chemistry
79.5K Visualizzazioni

Determinazione spettrofotometrica di una costante di equilibrio
General Chemistry
158.9K Visualizzazioni

Principio di Le Châtelier
General Chemistry
266.0K Visualizzazioni

Depressione del punto di congelamento per l'identificazione di un composto sconosciuto
General Chemistry
160.9K Visualizzazioni

Determinazione dell'equazione cinetica e dell'ordine di reazione
General Chemistry
196.5K Visualizzazioni

Utilizzo della calorimetria a scansione differenziale per misurare cambiamenti nell'entalpia
General Chemistry
44.8K Visualizzazioni

Complessi chimici di coordinazione
General Chemistry
91.8K Visualizzazioni