Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Recognition of Epidermal Transglutaminase by IgA and Tissue Transglutaminase 2 Antibodies in a Rare Case of Rhesus Dermatitis
W tym Artykule
Podsumowanie
Dermatitis herpetiformis (DH) is a chronic inflammatory condition characterized by an autoimmune reaction between IgA and epidermal transglutaminase (eTG). DH develops in a very small portion of gluten-sensitive and/or celiac patients. The results of this study indicate that DH can also develop in a rhesus monkey host with symptoms of idiopatic dermatitis.
Streszczenie
Tissue transglutaminase 2 (tTG2) is an intestinal digestive enzyme which deamidates already partially digested dietary gluten e.g. gliadin peptides. In genetically predisposed individuals, tTG2 triggers autoimmune responses that are characterized by the production of tTG2 antibodies and their direct deposition into small intestinal wall 1,2. The presence of such antibodies constitutes one of the major hallmarks of the celiac disease (CD). Epidermal transglutaminase (eTG) is another member of the transglutaminase family that can also function as an autoantigen in a small minority of CD patients. In these relatively rare cases, eTG triggers an autoimmune reaction (a skin rash) clinically known as dermatitis herpetiformis (DH). Although the exact mechanism of CD and DH pathogenesis is not well understood, it is known that tTG2 and eTG share antigenic epitopes that can be recognized by serum antibodies from both CD and DH patients 3,4.
In this study, the confocal microscopy examination of biopsy samples from skin lesions of two rhesus macaques (Macaca mulatta) with dermatitis (Table 1, Fig. 1 and 2) was used to study the affected tissues. In one animal (EM96) a spectral overlap of IgA and tTG2 antibodies (Fig. 3) was demonstrated. The presence of double-positive tTG2+IgA+ cells was focused in the deep epidermis, around the dermal papillae. This is consistent with lesions described in DH patients 3. When EM96 was placed on a gluten-free diet, the dermatitis, as well as tTG2+IgA+ deposits disappeared and were no longer detectable (Figs. 1-3). Dermatitis reappeared however, based on re-introduction of dietary gluten in EM96 (not shown). In other macaques including animal with unrelated dermatitis, the tTG2+IgA+ deposits were not detected. Gluten-free diet-dependent remission of dermatitis in EM96 together with presence of tTG2+IgA+ cells in its skin suggest an autoimmune, DH-like mechanism for the development of this condition. This is the first report of DH-like dermatitis in any non-human primate.
Protokół
1. Skin biopsy sample collection
- Prior to skin biopsy procedure, anesthetize animals intramuscularly with 2.5 mg/kg of tiletamine hydrochloride and zolazepam hydrochloride telazol mixture (Fort Dodge Animal Health, Fort Dodge, IA). Monitor the animals from administration of anesthetic until recumbency and then remove from their enclosure.
- Remove the hair from the skin area of interest utilizing an Oster Golden A5 Single Speed Veterinary Clipper with a size 40 blade (Oster Professional Products, McMinnville, TN) and aseptically prepare with alternating betadine scrub and alcohol. Secure a sterile fenestrated drape over the selected biopsy site.
- Using a sterile technique, place a 4.0 mm Miltex Punch Dermal Biopsy instrument (Miltex, York, PA) against the skin while rotating the instrument 180 degrees clockwise and counter clockwise with slight pressure until the biopsy punch transects through the dermal layers into the subcutaneous tissue. Remove the biopsy sample and grasp the transected portion of skin with forceps and free from the subcutaneous tissue.
- Close the skin defect with 3-0 nylon suture attached to a 3/8 circle cutting needle (Ethilon, Ethicon, Johnson & Johnson Medical Limited, Berkshire, UK) in a cruciate pattern. Give all animals 0.01 mg/kg buprenorphine hydrochloride (Hospira, Lake Forest, IL) intramuscularly for post operative analgesia.
2. Sample processing
- Work with skin biopsy samples from chronic dermatitis and healthy control rhesus macaques. Obtain two to three (4 mm in diameter) biopsy samples from each animal.
- Fix first sample in zinc formalin (Z-fix, Anatech Ltd., Battle Creek, MI) for 24 hours, wash in water for 30 min, wash briefly in 70% ethanol, and place into ASP300 Leica tissue processor (Leica Microsystems Inc., Buffalo Grove, KS) where tissue is dehydrated with ascending grades of 70%, 80%, 95% and 100% ethanol 48 min each (Fisher Scientific, Pittsburgh, PA), followed by two changes of xylene (Fisher).
- Embed in paraffin media (Fisher) for long-term storage at room temperature. Place at -20oC for 20 min prior to sectioning. Prepare 6 μm sections using a rotary microtome (HM325, Microm International, Waldorf, Germany). Place sections on charged slides (Fisher) and air dry at 60oC overnight. Stain with hematoxylin and eosin (H&E) standard method (described below).
- Fix second sample in 2% paraformaldehyde (USB Corp, Cleveland, OH) for 30 min at room temperature, wash three times in phosphate buffered saline (PBS, Gibco-Invitrogen, Carlsbad, CA), place in 30% sucrose (Fisher) for 4 hours, and embed in OCT freezing medium (Sakura Finetek, Torrence, CA). Keep at -80oC for 20 min prior to sectioning. Prepare 15 μm sections using the cryostat (HM560, Thermo Scientific, Kalamazoo, MI).
3. H&E staining
- Deparaffinize embedded sections through three changes of xylenes (Fisher) and rehydrate through graded ethanols: three changes of 100%, two changes of 95%, one change of 80% and distilled water, 2 min each.
- Stain with hematoxylin (Richard-Allan Scientific, Kalamazoo, MI) for one min and follow by a brief wash in running tap water.
- Counterstain with eosin (Sigma) for 6 min.
- Dehydrate stained tissues through 95% and absolute ethanol, two changes of 2 min each and then clear in three changes of xylenes, 3 min each.
- Mount coverglass using Cytoseal Mounting Medium (Richard-Allan Scientific) over the H&E-stained tissue sections.
4. Histopathological evaluation
Inspect H&E-stained biopsy skin sections under a light microscope using the 4 - 40x magnification and Leica microscope with a SPOT Insight digital camera (Digital Instruments Inc., Michigan, USA).
5. Immunofluorescent staining
- Work with OCT-embedded skin sections for immunofluorescent staining. Prior to staining, soak for 20 min in PBS containing 0.2% fish skin gelatin (FSG, Sigma) with 0.1%Triton X-100 (Sigma) to remove the OCT and to permeabilize the tissue.
- After washing the slides, incubate with 10% normal goat serum (NGS, Sigma), diluted in PBS-FSG for 1 hour, at room temperature, in a humidified slide chamber.
- In order to visualize the deposition of IgA and tTG2 antibodies, incubate slides with anti-rhesus IgA and tTG2 primary antibodies, diluted in 10% NGS-PBS-FSG for 1 hour.
- Wash with PBS–FSG–Triton X-100 (Sigma) for 10 min, followed by PBS-FSG for 10 min.
- Incubate with secondary, isotype-specific antibodies tagged with Alexa Fluor fluorochrome 488 (green), 568 (red) or 633 (blue) (Molecular Probes-Invitrogen) at 1:1,000 dilution. Use the ToPro-3 dye to visualize the nuclear DNA. Use the 10% NGS-PBS-FSG as an antibody diluent.
- Use the anti-quenching solution (Sigma) to mount the stained tissue sections.
6. Confocal imaging
Perform imaging with a Leica TCS SP2 True Confocal Laser Scanning Microscope (Leica, Wetzlar, Germany) equipped with three lasers (six lines for excitation available): an argon-krypton laser at 488 nm (green), a krypton laser at 568 nm (red) and a helium-neon laser at 633 nm (blue), that span from the visible to the far-red side of the spectrum. Record differential interference contrast (DIC) images for evaluation of non-labeled tissue architecture.
7. Representative Results
An example of positive recognition of eTG by both tTG2 and IgA antibodies is shown in Fig. 3A. Consistent with situation in human patients and due to much lower prevalence of DH-like dermatitis than CD-like gluten sensitivity also in rhesus macaques, most of the skin biopsy samples are expected to show the positive reaction with tTG2 antibodies but without the presence of tTG2+IgA+ double positive cells (Fig. 4). These DH-unrelated cases, including dermatitis cases of microbial origin, will reveal some single-positive IgA+ cells scattered beneath the epithelium but not spectral overlap between the tTG2 and IgA-labeled markers (Figs. 3B and 4). Therefore, it is important to maintain the order of suggested testing procedures, starting with clinical examination (Fig. 1), followed by histopathological evaluation (Fig. 2) and only if no other, more common dermatitis-causing agents can be identified, confocal microscopy examination of skin biopsies stained for tTG2 and IgA is warranted. Clinical and/or histopathological evaluations alone are not sufficient for positive diagnosis of this condition. In those cases when double-positive (tTG2+IgA+) cells are identified around the area of dermal papillae, DH-like dermatitis diagnosis can be issued. In such cases, withdrawal of dietary gluten and administration of gluten-free diet is recommended in order to alleviate the dermatitis symptoms.

Figure 1. Inguinal region of EM96 macaque before (A) and after (B) withdrawal of dietary gluten for 5 weeks. Reintroduction of dietary gluten coincided with the reappearance of dermatitis (C).
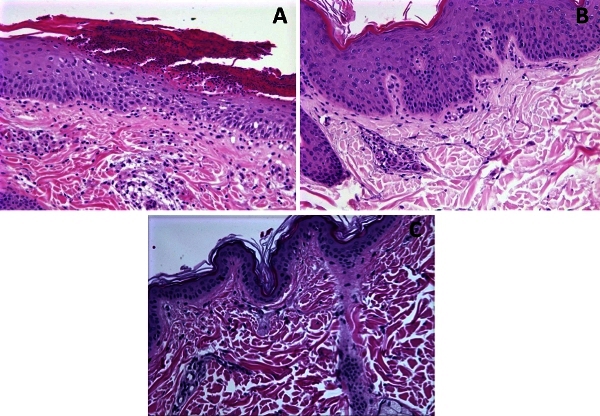
Figure 2. HE staining of EM96 macaque skin biopsies collected before (A) and after (B) withdrawal of dietary gluten for 9 weeks. A: Epithelium is thickened (7-15 layers of epithelial cells) with a thick superficial crust of hyperkeratotic cells infiltrated with degenerating neutrophils. Neutrophils appear also inside the epithelium and are mixed with moderate numbers of lymphocytes, plasma cells, and histiocytes around the adnexa in the upper dermis. B: Some hyperkeratosis is still present, epithelial thickness is normalized (4-7 layers) with minimal lymphoplasmacytic infiltration in the upper dermis. C: Normal abdominal skin from FR56 macaque, with no lymphoplasmacytic infiltration, is included for comparison. Magnification 20x.

Figure 3. Confocal microscopy images of EM96 macaque skin biopsies collected before (A) and after (B) withdrawal of dietary gluten for 9 weeks. A: IgA staining (green), tTG2 (red) and ToPro-3 nuclear DNA marker (blue). Co-localization of IgA and tTG2 appears in yellow around the area of dermal papilae. B: Skin sample taken after 9 weeks of gluten-free diet shows no IgA within the epithelium while some subepithelial IgA is still present (green), tTG2 (red) and ToPro-3 nuclear DNA marker (blue).
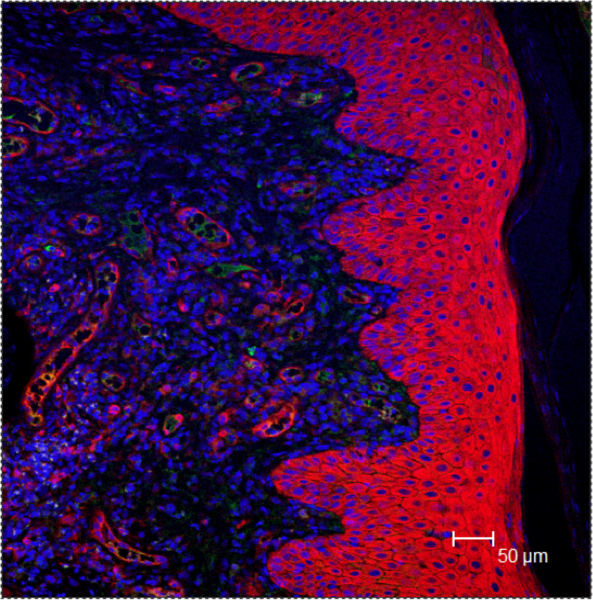
Figure 4. Confocal microscopy images of GI24 rhesus macaque skin biopsies collected while animal was exhibiting the symptoms of chronic (DH-unrelated) dermatitis. The IgA staining is in green, tTG2 in red and ToPro-3 nuclear DNA marker is in blue. The tTG2+ cells are present inside the epithelium, few IgA+ cells beneath the epithelium, while no extensive spectral overlap between the red and green markers is seen.
| Animal Tag | Sex [M/F] | Age [Years] | Clinical Symptoms | Plasma tTG2 antibodies | Diet [1,2]* |
| HJ71 | F | 1.3 | Healthy | - | 1 |
| HD13 | M | 1.4 | Healthy, occasional diarrhea | + | 1 |
| FR56 | M | 5.4 | Chronic idiopathic diarrhea | - | 1 |
| EM96 | F | 5.0 | Chronic dermatitis | +/- (borderline) | 1 and 2 |
| GI24 | F | 4.6 | Chronic dermatitis | - | 1 |
Table 1. TNPRC rhesus macaques used for collection of skin biopsies
* Diet 1 – regular monkey chow (gluten-containing); Diet 2 – gluten-free diet
Dyskusje
Our newly established non-human primate model of gluten sensitivity was recently used to study the intestinal permeability changes triggered by dietary gluten 5,6. Plasma tTG2 antibodies as well as intestinal tTG2+IgA+ deposits were detected in some gluten-sensitive macaques. Since we recently identified rare incidents of idiopathic dermatitis in colony rhesus macaques, where no usual (infectious/metabolic) etiology could be established, objective of this study was to evaluate if tTG2+IgA+ antibody deposits co...
Ujawnienia
No conflicts of interest declared.
Podziękowania
The authors would like to thank Amanda Tardo, Dr. Mostafa Bouljihad, Stephanie Feely, Carol Coyne, Dr. Erin Ribka, Dr. Pete Didier and Dorothy Kuebler for their technical assistance. This work was supported by NIH grants R01-DK076653 and 3R01-DK076653-02S1 to K.S. and the base operating grant of the TNPRC RR000164.
Materiały
| Name | Company | Catalog Number | Comments |
| Name of the reagent or equipment | Company | Catalogue Number / Product Code | Note |
| Anti-Human IgA-FITC * | Sigma, St. Louis, MO | F-5259 | 1:100 working dilution |
| Anti-Human tTG2 | Thermo Scientific, Kalamazoo, MI | MS-300-P1ABX | 1:100 working dilution |
| Anti-Mouse IgG1 ** | Invitrogen, Carlsbad, CA | A-21124 | 1:1,000 working dilution |
| ToPro-3 DNA probe *** | Invitrogen, Carlsbad, CA | T-3605 | 1:1,000 working dilution |
| Zinc Formalin (Z-fix) | Anatech Ltd., Battle Creek, MI | 171 | |
| OCT Freezing Medium | Sakura Finetek, Torrence, CA | 4583 | |
| Cytoseal Mounting Medium | Richard-Allan Scientific, Kalamazoo, MI | 8312-4 | |
| Anti-Quenching Solution | Sigma, St. Louis, MO | P 3130 | |
| Ethilon (Nylon Suture) | Ethicon, Johnson & Johnson, Berkshire, UK | 663G | |
| Miltex Punch Dermal Biopsy Instrument | Miltex, York, PA | NA | |
| Oster Golden A5 Single Speed Vet Clipper with a size 40 blade | Oster Products, McMinnville, TN | 78005-050 | |
| Leica Tissue Processor | Leica Microsystems Inc., Buffalo Grove, KS | ASP300 | |
| Rotary Microtome | Microm International, Waldorf, Germany | HM325 | |
| Cryostat | Thermo Scientific, Kalamazoo, MI | HM560 | |
| Confocal Microscope | Leica, Wetzlar, Germany | TCS SP2 |
* Fluorescein isothiocyanate
** Secondary (Alexa Fluor 568-conjugated) antibody used after tTG2 primary antibody
** Dye
Odniesienia
- Diosdado, B., Wijmenga, C. Molecular mechanisms of the adaptive, innate and regulatory immune responses in the intestinal mucosa of celiac disease patients. Expert. Rev. Mol. Diagn. 5 (5), 681-700 (2005).
- Schuppan, D., Dennis, M. D., Kelly, C. P. Celiac disease: epidemiology, pathogenesis, diagnosis, and nutritional management. Nutr. Clin. Care. 8 (2), 54-69 (2005).
- Sardy, M. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J. Exp. Med. 195 (6), 747-757 (2002).
- Rose, C. Autoantibodies against epidermal transglutaminase are a sensitive diagnostic marker in patients with dermatitis herpetiformis on a normal or gluten-free diet. J. Am. Acad. Dermatol. 61 (1), 39-43 (2009).
- Bethune, M. Transepithelial transport and enzymatic detoxification of gluten in gluten-sensitive rhesus macaques. PLoS ONE. 3 (3), e1857-e1857 (2008).
- Mazumdar, K. Visualization of transepithelial passage of the immunogenic 33-residue peptide from α-2 gliadin in gluten-sensitive macaques. PLoS ONE. 5 (4), e10228-e10228 (2010).
- Schuppan, D., Junker, Y., Barisani, D. Celiac disease: from pathogenesis to novel therapies. Gastroenterol. 137 (6), 1912-1933 (2009).
- Hunt, K. Newly identified genetic risk variants for celiac disease related to the immune response. Nature Genet. 40 (4), 395-402 (2008).
- Abadie, V. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 29, 493-525 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone