Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Purification and Aggregation of the Amyloid Precursor Protein Intracellular Domain
W tym Artykule
Podsumowanie
A method for large-scale purification of the APP intracellular domain (AICD) is described. We also describe methodology to induce in vitro AICD aggregation and visualization by atomic force microscopy. The methods described are useful for biochemical/structural characterization of the AICD and the effects of molecular chaperones on its aggregation.
Streszczenie
Amyloid precursor protein (APP) is a type I transmembrane protein associated with the pathogenesis of Alzheimer's disease (AD). APP is characterized by a large extracellular domain and a short cytosolic domain termed the APP intracellular domain (AICD). During maturation through the secretory pathway, APP can be cleaved by proteases termed α, β, and γ-secretases1. Sequential proteolytic cleavage of APP with β and γ-secretases leads to the production of a small proteolytic peptide, termed Aβ, which is amyloidogenic and the core constituent of senile plaques. The AICD is also liberated from the membrane after secretase processing, and through interactions with Fe65 and Tip60, can translocate to the nucleus to participate in transcription regulation of multiple target genes2,3. Protein-protein interactions involving the AICD may affect trafficking, processing, and cellular functions of holo-APP and its C-terminal fragments. We have recently shown that AICD can aggregate in vitro, and this process is inhibited by the AD-implicated molecular chaperone ubiquilin-14. Consistent with these findings, the AICD has exposed hydrophobic domains and is intrinsically disordered in vitro5,6, however it obtains stable secondary structure when bound to Fe657. We have proposed that ubiquilin-1 prevents inappropriate inter- and intramolecular interactions of AICD, preventing aggregation in vitro and in intact cells4. While most studies focus on the role of APP in the pathogenesis of AD, the role of AICD in this process is not clear. Expression of AICD has been shown to induce apoptosis8, to modulate signaling pathways9, and to regulate calcium signaling10. Over-expression of AICD and Fe65 in a transgenic mouse model induces Alzheimer's like pathology11, and recently AICD has been detected in brain lysates by western blotting when using appropriate antigen retrieval techniques12. To facilitate structural, biochemical, and biophysical studies of the AICD, we have developed a procedure to produce recombinantly large amounts of highly pure AICD protein. We further describe a method for inducing the in vitro thermal aggregation of AICD and analysis by atomic force microscopy. The methods described are useful for biochemical, biophysical, and structural characterization of the AICD and the effects of molecular chaperones on AICD aggregation.
Protokół
1. Expression of Recombinant APP Intracellular Domain (AICD)
- Transform E. coli strain BL21 with human AICD (residues 649-695 of APP, neuronal isoform numbering) cloned into vector pGEX-4T-1 (GE Healthcare). This vector will express AICD as the C-terminal moiety of a fusion protein of glutathione-S-transferase (GST). This vector also encodes a thrombin cleavage sequence to facilitate removal of the GST moiety. Details of cloning AICD into pGEX-4T-1 can be found in our previous publication4.
- From a single colony, inoculate 10 ml of LB broth with ampicillin (100 μg/ml), and incubate overnight at 37 °C with vigorous shaking.
- The following morning, inoculate 400 ml LB/ampicillin in a one-liter flask with 5 ml of the overnight culture.
- Incubate at 37 °C with vigorous shaking until the optical density at 600 nm has reached 0.4 to 0.6. This will normally take 2 to 2.5 hr.
At this point it may be desirable to take out a small sample (~10 μl) to follow the purification by SDS-PAGE (see Figure 1A,B). Each subsequent purification step should also have a small aliquot removed for analysis by SDS-PAGE.
- Induce expression of GST-AICD by adding 0.4 mM of isopropyl-beta-D-thiogalactopyranoside (IPTG). Incubate at 37 °C with vigorous shaking for 5 hr.
- Centrifuge the cells at 6,000 x g for 15 min at 4 °C. The cell pellet can be stored at -80 °C for several months at this point of the procedure.
2. Purification of GST-AICD
- Prepare 50 ml lysis buffer as follows: 1 mM dithiothreitol, 1mM ethylenediaminetetraacetic acid (EDTA), 0.1% Triton-X100, 1 mM phenylmethylsulfonyl fluoride (PMSF), Complete protease inhibitor cocktail (Roche Applied Science), and phosphate buffered saline. Chill on ice.
- Thaw bacterial pellet (if previously frozen) on ice.
At this point, it is critical that all steps are performed at 4 °C to limit proteolysis. All tubes, buffers, and other reagents should be pre-chilled. If using a French press or emulsifier for lysis, these should be pre-chilled as well.
- Thoroughly resuspend the bacterial pellet in 20 ml of lysis buffer. A combination of vortexing and trituration with 10 ml pipette on ice will ensure complete resuspension of the pellet.
- Pass the resuspended bacterial cells through an emulsifier (e.g., Avestin EmulsiFlex-C3) or French pressure cell, pre-chilled to 4 °C at 30,000 psi. Pass the lysate through the emulsifier a second time. Collect the lysate into a pre-chilled 50 ml tube.
The main advantage of these two instruments is that they minimize heating of the sample. The commonly used technique of sonication generates significant heat at the tip of the sonication probe and can result in aggregation of recombinant proteins.
- Centrifuge the lysate at 15,000 x g for 30 min. Separate and keep the supernatant into a pre-chilled 50 ml tube.
- Add 500 μl of a 50% slurry of glutathione-agarose (Sigma-Aldrich). Rotate for 30 min at 4 °C. While the sample is rotating, make fresh elution buffer: 50 mM Tris 7.8, 0.1% Triton-X100, 0.5 mM dithiothreitol, 10 mM reduced glutathione.
The only step of the protocol which is performed at room temperature is the elution step. Thus, the elution buffer is kept at room temperature. Elution buffer should be prepared fresh.
- Pour the lysate and slurry into a disposable 5" polystyrene chromatography column with coarse (90-130 μm) filters (Evergreen Scientific). Wash the column 5 times with 3 ml of phosphate buffered saline.
- Cap the bottom of the column and add 500 μl of room temperature elution buffer. Cap the top of the column and rotate for 5 min at room temperature. Collect the eluate into a chilled 1.5 ml microfuge tube. Repeat the elution two more times (1.5 ml total eluate).
- Dialyze the eluate in a 10,000 molecular weight cutoff dialysis membrane (e.g., Thermo Scientific Slide-A-Lyzer dialysis cassettes) in 4 liters of phosphate buffered saline overnight at 4 °C. Dialyze against an additional 4 liters of phosphate buffered saline at 4 °C for 1 hr the following day.
- Remove the protein from the dialysis cassette and perform a protein quantification assay to determine yield of GST-AICD. A typical purification from 400 ml of culture will result in yields of >20 mg of purified protein at concentrations of 10-20 mg/ml. Aliquot the protein into 200 μl aliquots and freeze at -80 °C.
3. Thrombin Cleavage of GST-AICD and Purification of AICD
- Thaw 200 μl of purified GST-AICD and add 20 μl of 1 U/μl thrombin. Incubate overnight at 37 °C.
The quality and purity of thrombin varies considerably among manufacturers, and can be a significant source of contamination. We use thrombin from GE Healthcare (see table of reagents). After overnight incubation, >95% of the fusion protein should be cleaved (Figure 1B).
- After overnight cleavage with thrombin, it is likely that a fraction of the liberated AICD underwent aggregation and the solution may appear cloudy. Clear the aggregated material by centrifugation at 20,000 x g for 30 min at 4 °C. Remove the supernatant into a pre-chilled microfuge tube.
- Add 50 μl of 50% glutathione-agarose slurry and 50 μl 50% p-aminobenzamidine-agarose slurry to remove GST and thrombin, respectively. Incubate with rotation for 5 min at room temperature.
- Centrifuge briefly to sediment the agarose beads, and remove the supernatant into a fresh tube. Extract the GST four more times with glutathione-agarose. A single extraction with p-aminobenzamidine-agarose is sufficient to remove the 20 U of thrombin.
- Perform a protein quantitation assay on 5 μl of the purified AICD. Typical yields from a 400 ml culture are 50-100 μg at a concentration of 0.2 to 0.5 mg/ml. Higher yields are possible by cleaving larger volumes of GST-AICD.
Purified AICD should be prepared fresh for biochemical/biophysical characterization. Unused material should be discarded. It is also possible to concentrate the AICD by lyophilizing the sample and resuspending in a smaller volume of buffer6. If detecting AICD requires blotting (such as after a filter trap or dot blot assay), antigen retrieval on the membrane should be performed as described by Pimplikar and Suryanarayana12.
4. AICD Aggregation for Atomic Force Microscopy (AFM)
- Dilute freshly prepared AICD in phosphate buffered saline to a concentration of 0.1 mg/ml in a final volume of 15 μl. At this point, various compounds and/or proteins may be added to determine if they inhibit AICD aggregation. For example, we have shown that equimolar concentrations of ubiquilin-1 prevent aggregation of AICD in this assay4.
It is also possible to aggregate larger reaction volumes for biochemical experiments such as filter trap assays and light scattering4. We have had success performing filter trap assays on reaction mixtures as large as 400 μl with AICD diluted to concentrations as low as 1 μM.
- Induce thermal aggregation by incubating the samples at 43 °C with shaking at 800 rpm in an Eppendorf Thermomixer for 48 hr. This temperature was chosen based upon chaperone assays which examine the thermal aggregation of the model chaperone substrate citrate synthase4,13.
Thermal aggregation is a commonly used method to induce the structural transition of a protein from a native to a non-native structure (which is usually beta sheet rich) which results in the formation of intermolecular aggregates. Thermal aggregation is also used to examine the function of molecular chaperones, which shield hydrophobic segments from forming inappropriate intermolecular interactions (and thus retard or prevent thermal aggregation).
- Dilute the sample 20-fold in deionized water and spot 2 μl onto freshly cleaved mica. Dry the sample in a dessicator overnight. It will be necessary to empirically determine the optimal dilution for imaging, and thus several dilutions ranging from 10- to 100-fold would be prudent.
- Visualize the aggregated AICD using the AFM operating in tapping mode14. The cantilevers used are gold-coated micro sharp nitride levers (MSNL, Bruker) with a nominal tip radius of 2 nm. Typical tapping amplitudes during imaging are 10-20 nm at the resonance frequency of the cantilevers (30-50 kHz).
We found that image quality and contrast of different protein aggregates depended on the applied force and the duration of the experiment. To minimize sample perturbation by the tip, we maintained the applied force relatively low by keeping a set-point ratio above 95% and limiting the scanning of each sample to 5-10 min. Image processing was performed with WSxM software (Nanotec). Standard image processing consisted of plane subtraction and flattening. Our laboratories use a "home-built" AFM15 interfaced to a commercial scanning probe microscope control system (Nanotec).
5. Representative Results
The expression and relative enrichment of GST-AICD is shown in Figure 1A. After lysis, the majority of the fusion protein is present in the soluble fraction, and therefore extraction from inclusion bodies is not required. The material eluted from the glutathione agarose column is >95% pure. In the preparation shown in Figure 1, the concentration of protein eluted from the column was 14.5 mg/ml, and the yield was 22 mg (from a starting culture of 400 ml). Cleavage of 200 μl of this preparation overnight with 20 U of thrombin resulted in almost 100% cleavage of the fusion protein (Figure 1B). The amount of thrombin used is low enough as to not be detectable by Coomassie blue staining (see "Cleaved" lane, Figure 1B). Removal of the thrombin and GST resulted in some loss of material, however it was >90% pure with a yield in this prep of 70 μg of AICD at a concentration of 0.35 mg/ml. Figure 1C shows the flow chart for aggregation of AICD and subsequent analysis. AICD aggregates imaged by AFM are typically spheroid/amorphous, and range in size from 50 to 100 nm (Figure 1D,F). Aggregated material is not detectable when an equimolar amount of the chaperone ubiquilin-1 is added to the aggregation reaction (Figure 1E)4.
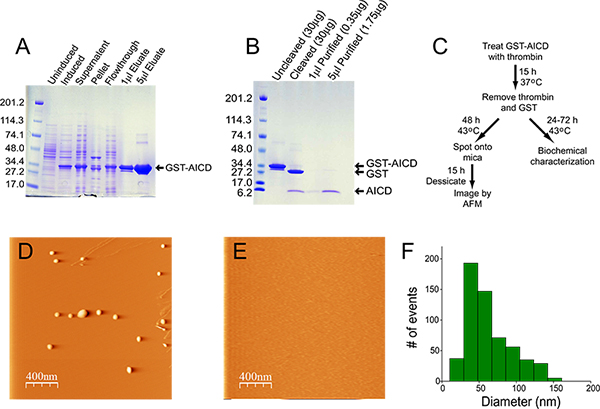
Figure 1. Purification and aggregation of AICD. A) Coomassie blue staining of samples collected at each step of the purification process. For the uninduced and induced samples, 20 μl of total bacteria were run on the gel. For the supernatant fraction, 5 μl of lysate (from a total volume of 20 ml) were run on the gel. For the pellet fraction, the pellet was resuspended in 20 ml of lysis buffer, and 5 μl of this material were sonicated in a water bath sonicator for 30 min at 4 °C prior to loading onto the gel. For the flowthrough, 5 μl were run on the gel. The amounts of eluate loaded onto the gel are indicated. B) Coomassie blue stained gel of uncleaved and cleaved GST-AICD (2 μl) and 1 and 5 μl purified AICD. C) Flowchart of AICD purification, aggregation, and analysis. D,E) Representative AFM image of thermally aggregated AICD in the absence (D) and presence (E) of the molecular chaperone ubiquilin-1. F) Histogram of the size distribution of AICD aggregates.
Dyskusje
In this protocol we have outlined a procedure for obtaining highly pure AICD for structural, biophysical, and biochemical analyses. This procedure does not require sophisticated chromatography equipment and is therefore accessible to most laboratories. Other groups have purified AICD5-7,16, including GST-AICD17-19, for biochemical/structural analyses. Disadvantages to previous protocols include poor solubility of AICD16, less than ideal purity17, and the requirement for size ex...
Ujawnienia
No conflicts of interest declared.
Podziękowania
The authors would like to thank Dr. Hui Zheng (Baylor College of Medicine) for the APP cDNA. This work was funded by NIH grants R21AG031948 (D.B., J.M.B.), F30AG030878 (E.S.S.), R01DK073394 (AFO), the John Sealy Memorial Endowment Fund for Biomedical Research (AFO), and the Jean C. and William D. Willis Neuroscience Research Endowment (E.S.S.). J.M.B. is a scholar in the Translational Research Scholar Program and a member of the University Of Texas Medical Branch Claude E. Pepper Older Americans Independence Center (supported by NIH Grants UL1RR029876 and P30-AG-024832, respectively).
Materiały
| Name | Company | Catalog Number | Comments |
| Name of the reagent | Company | Catalogue number | Comments |
| pGEX-4T-1 | GE Healthcare | 28-9545-49 | |
| Thrombin | GE Healthcare | 27-0846-01 | |
| Ampicillin | Fisher Scientific | BP1760 | |
| Bradford protein assay reagent | Bio-Rad | 500-0002 | |
| Coomassie blue | Bio-Rad | 161-0786 | |
| IPTG ( isopropyl-beta-D thiogalactopyranoside) | Sigma-Aldrich | I6758 | |
| Glutathione-agarose | Sigma-Aldrich | G4510 | |
| p-aminobenzamidine-agarose | Sigma-Aldrich | A7155 | |
| Complete protease inhibitor cocktail | Roche | 11836170001 | |
| Slide-A-Lyzer dialysis cassettes | Thermo Scientific | 66380 | |
| Chromatography columns | Evergreen Scientific | 208-3367-050 | |
| Emulsifier | Avestin, Inc | EmulsiFlex-C3 | Highly recommended |
| Eppendorf Thermomixer | Eppendorf | 022670107 | |
| Mica Disks | Ted Pella | 50-12 | |
| AFM cantilevers | Bruker | MSNL-10 | |
| WSxM software | Nanotec | N/A | Free download |
Odniesienia
- De Strooper, B., Vassar, R., Golde, T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nature reviews. Neurology. 6, 99-107 (2010).
- Chang, K. A., Suh, Y. H. Possible roles of amyloid intracellular domain of amyloid precursor protein. BMB reports. 43, 656-663 (2010).
- McLoughlin, D. M., Miller, C. C. The FE65 proteins and Alzheimer's disease. J. Neurosci. Res. 86, 744-754 (2008).
- Stieren, E. S. Ubiquilin-1 is a molecular chaperone for the amyloid precursor protein. J. Biol. Chem. 286, 35689-35698 (2011).
- Ramelot, T. A., Nicholson, L. K. Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. J. Mol. Biol. 307, 871-884 (2001).
- Ramelot, T. A., Gentile, L. N. Transient structure of the amyloid precursor protein cytoplasmic tail indicates preordering of structure for binding to cytosolic factors. Biochem. 39, 2714-2725 (2000).
- Radzimanowski, J. Structure of the intracellular domain of the amyloid precursor protein in complex with Fe65-PTB2. EMBO Rep. 9, 1134-1140 (2008).
- Ohkawara, T., Nagase, H., Koh, C. S., Nakayama, K. The amyloid precursor protein intracellular domain alters gene expression and induces neuron-specific apoptosis. Gene. 475, 1-9 (2011).
- von Rotz, R. C. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J. Cell Sci. 117, 4435-4448 (2004).
- Hamid, R. Amyloid precursor protein intracellular domain modulates cellular calcium homeostasis and ATP content. J. Neurochem. 102, 1264-1275 (2007).
- Ghosal, K., Stathopoulos, A., Pimplikar, S. W. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS ONE. 5, e11866 (2010).
- Pimplikar, S. W., Suryanarayana, A. Detection of APP intracellular domain in brain tissue. Met. Molecul. Biol. 670, 85-91 (2011).
- Buchner, J., Grallert, H., Jakob, U. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Met. Enzymol. 290, 323-338 (1998).
- Hansma, H. G. Recent advances in atomic force microscopy of DNA. Scanning. 15, 296-299 (1993).
- Valbuena, A. Quasi-simultaneous imaging/pulling analysis of single polyprotein molecules by atomic force microscopy. Rev. Sci. Instrum. 78, 113707 (2007).
- Radzimanowski, J., Beyreuther, K., Sinning, I., Wild, K. Overproduction, purification, crystallization and preliminary X-ray analysis of human Fe65-PTB2 in complex with the amyloid precursor protein intracellular domain. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 64, 409-412 (2008).
- Chen, T. Y., Liu, P. H., Ruan, C. T., Chiu, L., Kung, F. L. The intracellular domain of amyloid precursor protein interacts with flotillin-1, a lipid raft protein. Biochem. Biophys. Res. Commun. 342, 266-272 (2006).
- Kim, M. Y. Regulation of Notch1 signaling by the APP intracellular domain facilitates degradation of the Notch1 intracellular domain and RBP-Jk. J. Cell Sci. 124, 1831-1843 (2011).
- Lazarov, O. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J. Neurosci. 25, 2386-2395 (2005).
- Kakuda, N. Equimolar production of amyloid beta-protein and amyloid precursor protein intracellular domain from beta-carboxyl-terminal fragment by gamma-secretase. J Biol. Chem. 281, 14776-14786 (2006).
- Gosal, W. S., Myers, S. L., Radford, S. E., Thomson, N. H. Amyloid under the atomic force microscope. Protein Pept. Lett. 13, 261-270 (2006).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone