Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Activating Molecules, Ions, and Solid Particles with Acoustic Cavitation
W tym Artykule
Podsumowanie
Acoustic cavitation in liquids submitted to power ultrasound creates transient extreme conditions inside the collapsing bubbles, which are the origin of unusual chemical reactivity and light emission, known as sonoluminescence. In the presence of noble gases, nonequilibrium plasma is formed. The "hot" particles and the photons generated by collapsing bubbles are able to excite species in solution.
Streszczenie
The chemical and physical effects of ultrasound arise not from a direct interaction of molecules with sound waves, but rather from the acoustic cavitation: the nucleation, growth, and implosive collapse of microbubbles in liquids submitted to power ultrasound. The violent implosion of bubbles leads to the formation of chemically reactive species and to the emission of light, named sonoluminescence. In this manuscript, we describe the techniques allowing study of extreme intrabubble conditions and chemical reactivity of acoustic cavitation in solutions. The analysis of sonoluminescence spectra of water sparged with noble gases provides evidence for nonequilibrium plasma formation. The photons and the "hot" particles generated by cavitation bubbles enable to excite the non-volatile species in solutions increasing their chemical reactivity. For example the mechanism of ultrabright sonoluminescence of uranyl ions in acidic solutions varies with uranium concentration: sonophotoluminescence dominates in diluted solutions, and collisional excitation contributes at higher uranium concentration. Secondary sonochemical products may arise from chemically active species that are formed inside the bubble, but then diffuse into the liquid phase and react with solution precursors to form a variety of products. For instance, the sonochemical reduction of Pt(IV) in pure water provides an innovative synthetic route for monodispersed nanoparticles of metallic platinum without any templates or capping agents. Many studies reveal the advantages of ultrasound to activate the divided solids. In general, the mechanical effects of ultrasound strongly contribute in heterogeneous systems in addition to chemical effects. In particular, the sonolysis of PuO2 powder in pure water yields stable colloids of plutonium due to both effects.
Wprowadzenie
The use of power ultrasound in numerous industrial and research areas, such as the cleaning of solid surfaces, degassing of liquids, material sciences, environmental remediation, and medicine, has received much attention during the last decade 1. The ultrasonic treatment increases the conversion, improves the yield, and initiates the reactions in homogeneous solutions as well as in heterogeneous systems. It is generally accepted that the physical and chemical effects of ultrasonic vibrations in liquids arise from acoustic cavitation or, in other words, to the implosive collapse of microbubbles in fluids irradiated with power ultrasound 2. Violent implosion of the cavitation bubble generates transient extreme conditions in the gas phase of the bubble, which are responsible for the formation of chemically active species and sonoluminescence. Nevertheless, debate still continues over the origin of such extreme conditions. Spectroscopic analysis of the sonoluminescence helps to better understand the processes occurring during the bubble collapse. In water, saturated with noble gases, the sonoluminescence spectra are composed from OH(A2Σ+-X2Πi), OH(C2S+-A2S+) bands and a broad continuum ranging from UV to NIR part of the emission spectra 3. Spectroscopic analysis of OH(A2Σ+-X2Πi) emission bands revealed formation of nonequilibrium plasma during sonolysis of water 4, 5. At low ultrasonic frequency, weakly excited plasma with Brau vibrational distribution is formed. By contrast, at high-frequency ultrasound, the plasma inside collapsing bubbles exhibits Treanor behavior typical for strong vibrational excitation. The vibronic temperatures (Tv, Te) increase with ultrasonic frequency indicating more drastic intrabubble conditions at high-frequency ultrasound.
In principal, each cavitation bubble can be considered as a plasma chemical microreactor providing highly energetic processes at almost room temperature of the bulk solution. The photons and the "hot" particles produced inside the bubble enable to excite the non-volatile species in solutions thus increasing their chemical reactivity. For example, the mechanism of ultrabright sonoluminescence of uranyl ions in acidic solutions is influenced by uranium concentration: photons absorption/re-emission in diluted solutions, and excitation via collisions with "hot" particles contributes at higher uranyl concentration 6. Chemical species produced by cavitation bubbles can be used for the synthesis of metallic nanoparticles without any templates or capping agents. In pure water sparged with argon, the sonochemical reduction of Pt(IV) occurs by hydrogen issued from sonochemical water molecules splitting yielding monodispersed nanoparticles of metallic platinum 7. Sonochemical reduction is accelerated manifold in the presence of formic acid or Ar/CO gas mixture.
Many previous studies have shown the advantages of ultrasound to activate the surface of divided solids due to the mechanical effects in addition to chemical activation 8,9. Small solid particles that are much less in size than the cavitation bubbles do not perturb the symmetry of collapse. However, when a cavitation event occurs near big aggregates or near extended surface the bubble implodes asymmetrically, forming a supersonic microjet leading to the cluster disaggregating and to the solid surface erosion. Ultrasonic treatment of plutonium dioxide in pure water sparged with argon causes formation of stable nanocolloids of plutonium(IV) due to both physical and chemical effects 10.
Protokół
1. Measurement of Uranium Sonoluminescence
The thermostated cylindrical sonoreactor is mounted on top of a high-frequency transducer providing 203 or 607 kHz ultrasound. Ultrasonic irradiation with low frequency ultrasound of 20 kHz is performed with a 1-cm2 titanium horn placed reproducibly on top of the reactor. The emission spectra are recorded in the range 230–800 nm using a spectrometer coupled to a liquid-nitrogen cooled CCD camera. Hydrogen in the outlet gas is measured simultaneously with the spectroscopic study using a quadrupole mass spectrometer (MS).
- Prepare the sonoreactor by tightly attaching the glass part onto the high-frequency transducer and the Teflon lid holding the 20 kHz horn onto the glass part. Put the sonoreactor on the translation stage and adjust its position so as to image with the two mirrors the center of the reactor onto the entrance slit of the emission spectrometer.
- Prepare uranyl solutions in perchloric acid by dissolving weighted UO3 samples, provided by CETAMA/CEA France, in a minimal volume of concentrated HClO4 under heating. Adjust then the volume of solution with diluted HClO4. To prepare uranyl solutions in H3PO4 dissolve UO3 samples in concentrated HClO4, evaporate the obtained solution to the wet salts and dissolve the latter in the desired volume of 0.5 M H3PO4.
- Put the solution to study into the sonoreactor. Tightly replace the 20 kHz horn. Add thermocouple and inlet gas tube onto the sonoreactor and connect the outlet gas tube to the entrance of the mass spectrometer.
- Put on the cryostat at ~0-1 °C. Let argon bubble in the solution at a flow rate of 100 ml/min for at least 30 min and start following Ar and H2 MS signals.
- When MS signals are constant, switch on the ultrasonic generator (either the high-frequency one, at 60-80 W, or the 20 kHz one, at 35 W) and wait approximately 20 min until a steady-state temperature of about 10 °C is reached inside the sonoreactor. The H2 MS signal should increase, indicating cavitation and water sonolysis.
- Close the light-tight box around the sonoreactor and start measuring sonoluminescence spectra, each during 300 sec to ensure good signal intensity. For each wavelength interval make three spectra to increase the signal to noise ratio and put second-order-light filter when necessary.
- After measuring the SL spectra, switch off the ultrasonic generator and keep measuring MS signals until a nice baseline is reached. At the same time, measure emission spectra in the absence of US that will allow to correct SL spectra for parasite light.
2. Sonochemical Reduction of Pt(IV) in Aqueous Solutions
- Prepare a 5 g/L Pt(IV) solution starting from H2PtCl6·6H2O salt. Remarks: platinum salts are light and moisture sensitive. Keep the remaining salt under inert atmosphere and if possible, carry out the weighting procedure within a nonreactive gas atmosphere glove box.
- Under a fume hood, set up a 50 ml airtight glass reactor equipped with a double jacket (Figure 6).
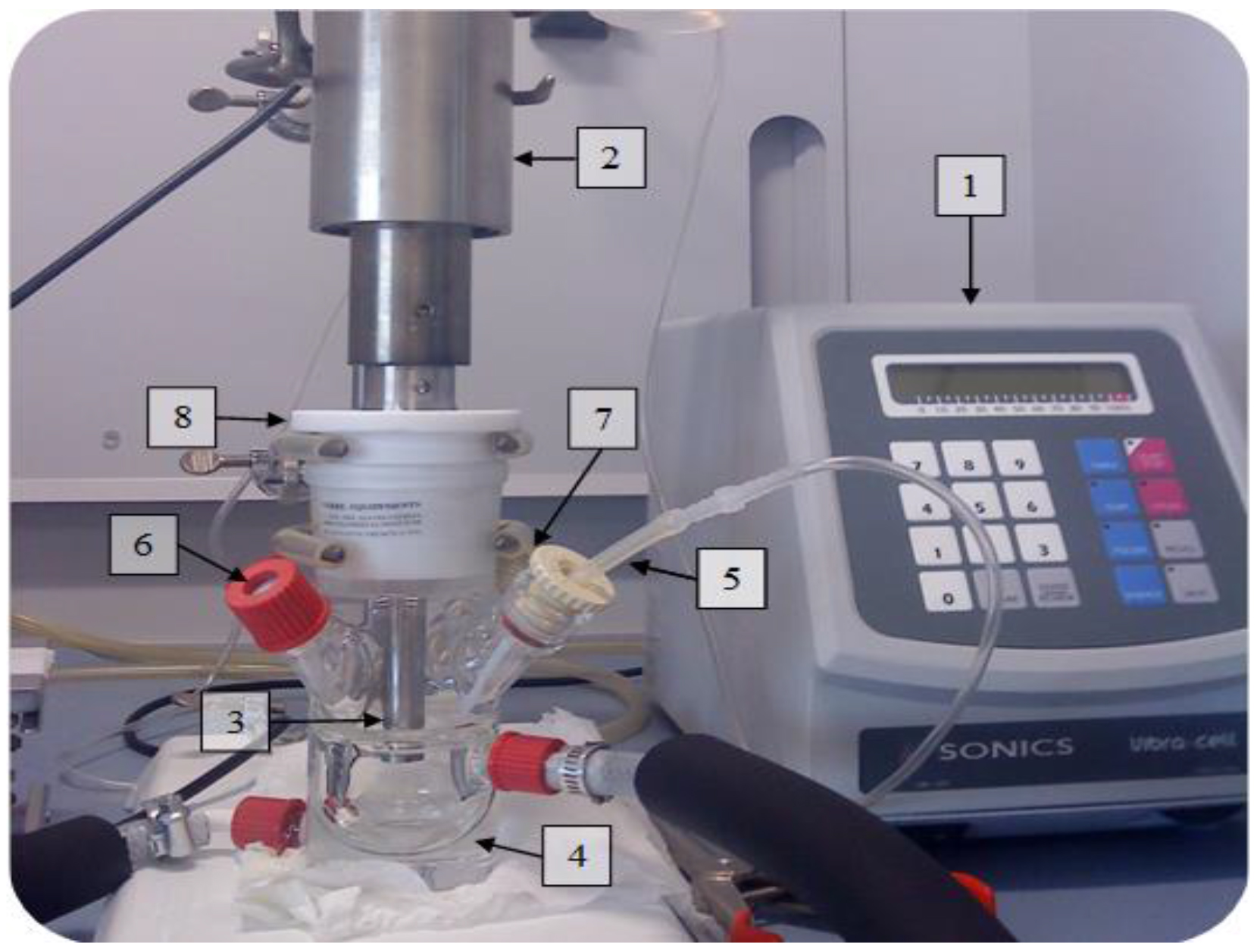
Figure 6: Experimental set-up for Pt(IV) sonochemical reduction at 20 kHz. 1. Ultrasonic generator of 20 kHz ultrasound with 750 W of maximal electric power, 2. Piezoceramic transducer, 3. Titanium horn, 4. Thermostated reactor, 5. Gas inlet, 6. Sample outlet, 7. Thermocouple, 8. PTFE ring. - Equip the reactor with a Pt-100 thermocouple, a septum, a PTFE gas inlet and also a gas outlet with flow meters calibrated within the range of 100 ml/min. Connect the gas outlet to a water trap (molecular sieves) and finally to a gas mass spectrometer. CAUTION: Be sure to evacuate the gas within the fume hood since CO is a very harmful compound. A CO gas detector in the laboratory is mandatory.
- At the top of the reactor, fix a 1 cm² titanium probe with a piezoelectric transducer supplied by a 20 kHz generator. Ensure that the sonotrode tip is at around 2 cm from the bottom of the reactor.
- Prior to experiments, start the chiller and set the temperature to -18 °C. In the meantime, introduce 50 ml of deionized water within the reactor and make the Ar/CO (10%) gas bubbling deep within the solution with a flow rate around 100 ml/min. Ensure that there is no major leakage by checking the gas outlet flow rate. Be sure that the sonotrode tip is 1 to 2 centimeters below the surface of the liquid and start the gaseous products monitoring.
- After 10 to 15 min, fix the gas inlet slightly below the liquid surface and once the chiller reaches the setup temperature, start the ultrasonic irradiation with an acoustic power of 17 W/ml.
- After 15 to 20 min of ultrasonic irradiation, check that the temperature reaches a steady state around 40 °C. If not, change the chiller settings to meet this requirement.
- Take a precise amount of the H2PtCl6 solution with the help of a syringe equipped with a stainless steel needle. Carefully introduce the needle through the septum and inject the solution within the cavitation zone below the sonotrode tip. Wash out the syringe by gently pumping the solution in and out and finally take a 1 ml sample. Repeat the sampling procedure at regular time intervals of 15 to 30 min.
- Measure the total concentration evolution of Pt ions in solution by ICP-OES analysis after dilution of the aliquots in 0.3 M HNO3. In the meantime, determine the amount of Pt(IV) ions within the system by following the 260 nm band in UV/Vis spectroscopy.
- As soon as no platinum ions can be detected in solution, switch off the ultrasonic irradiation, turn off the gas bubbling and the chiller. Take the platinum nanoparticle suspension out of the reactor.
- Prior to TEM analysis, try to centrifuge the suspension at high rotation speed (20,414 x g) for at least 20 min. Carefully remove the supernatant and store the deposit after drying at room temperature under vacuum or leave it within a small amount of water.
- Some samples can be very difficult to concentrate and can need longer centrifugation time. If it is not successful, use this procedure only to separate the platinum nanoparticles from the bigger titanium particles released in solution during the ultrasonic irradiation and then keep the supernatant this time.
- Disperse one drop of supernatant or few milligrams of dried products in absolute ethanol or isopropanol. Deposit one drop of the suspension on a carbon coated copper grid and proceed to the HRTEM analysis after total evaporation of the solvent.
3. Sonochemical Synthesis of Plutonium Colloids
In Marcoule, the ATALANTE facility is equipped with several hot labs and shielded cell lines dedicated to the research and development for nuclear fuel cycle. One of the glove boxes is devoted to the study of the sonochemical reactions of actinides.
- Suspend 200 mg of PuO2 (SBET = 13.3 m2/g) in 50 ml of pure water in the sonochemical reactor located in the glove box.
- Equip the reactor with the tight Teflon ring and the 20 kHz ultrasonic probe. Before each experiment, screw a new tip to ensure maximal effect of cavitation and avoid the accumulation of titanium particles in solution resulting from the tip erosion.
- Set the temperature of the cryostat (Huber CC1) situated outside the glove box low enough to manage the temperature increase in solution after the ultrasound will be switched-on. Note that the cooling system is equipped with a heat exchanger to avoid radioactive contamination outside the barrier. Insert the tight thermocouple into the cell to control the temperature of the solution.
- Allow bubbling the solution with pure argon 20 min before sonication (100 ml/min). Note that the Ar bubbling will be applied during the whole sonication experiments to ensure the maximal effects of acoustic cavitation.
- Set the ultrasonic generator to the appropriate amplitude (~30%) in order to obtain the required acoustic power Pac (17 W/cm2) delivered to the solution. Note that the acoustic power is previously measured using the thermal probe method 22. Using the appropriate conditions, the accumulation of hydrogen peroxide in solution (resulting from the combination of hydroxyl radicals induced by the homolytic dissociation of sonicated water molecules) is previously measured in pure water to calibrate the system and allow the reproducibility of the experiment.
- Switch-on the ultrasonic generator and sonicate the PuO2 solution. Adjust the cryostat settings to obtain a temperature of 30 °C in the solution.
- Once the colloids are formed (after 5-12 hr of irradiation), switch-off the ultrasonic generator, transfer the solution to centrifugation tube, and centrifuge during 10 min (22,000 x g) in order to remove the solid phase.
- UV-Vis spectrometer can thereafter be used for direct analysis and characterization of Pu colloids. During sonication, the kinetics of H2O2 accumulation in solution under ultrasound irradiation can also be measured by the colorimetric method at 410 nm (ε = 780 cm-1M-1) after diluting 500 µl of sampled solution with 500 µl TiOSO4 (2 x 10-2 M in 2 M HNO3 – 0.01 M [N2H5][NO3]) followed by centrifugation.
Wyniki
Uranyl ion sonoluminescence is extremely weak in HClO4 solutions: though typical light absorption by UO22+ ions is observed below 500 nm, emission lines from excited (UO22+)* (centered at 512 nm and 537 nm) are hardly seen (Figure 1). The SL of UO22+ is quenched. This quenching can be attributed to reduction of the excited uranyl ion by a coordinated water molecule 11-13:
(...
Dyskusje
The most critical parameters for successful observation of sonoluminescence and sonochemistry are: 1) rigorous control of the saturating gas and the bulk temperature during sonication, 2) careful selection of ultrasonic frequency, 3) using an optimal composition of sonicated solution to prevent quenching.
The kinetics of the sonochemical reactions as well as the intensity of sonoluminescence is very sensitive to the temperature of solution submitted to ultrasound: in contrast to the kinetics o...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors would like to acknowledge the French ANR (grant ANR-10-BLAN-0810 NEQSON) and CEA/DEN/Marcoule.
Materiały
| Name | Company | Catalog Number | Comments |
| 20 kHz Ultrasound Generator | Sonics Vibracell | ||
| Multifrequency Generator AG 1006 | T&C Power Conversion | ||
| Cryostat RE210 | Lauda | ||
| Spectrometer SP 2356i | Roper Scientific | ||
| CCD camera SPEC10-100BR cooled with liquid nitrogen | Roper Scientific | ||
| Quadrupole mass-spectrometer PROLAB 300 | Thermoscientific | ||
| Centrifuge Sigma 1-14 | Sigma-Aldrich | ||
| H2PtCl6 6H2O | Sigma-Aldrich | ||
| Ar; Ar/CO gases | Air Liquid | ||
| Uranium and Plutonium compounds | Prepared in the laboratories of Marcoule Research Center | ||
| Perchloric acid | Sigma-Aldrich | ||
| Phosphoric acid | Sigma-Aldrich | ||
| Formic acid | Sigma-Aldrich |
Odniesienia
- Mason, T. J., Lorimer, J. P. Applied Sonochemistry. The Uses of Power Ultrasound in Chemistry and Processing. Wiley-VCH Verlag GmbH. , (2002).
- Suslick, K. S. . Ultrasound: Its Chemical, Physical, and Biological Effects. , (1988).
- Pflieger, R., Brau, H. -. P., Nikitenko, S. I. Sonoluminescence from OH(C2Σ+) and OH(A2Σ+) Radicals in Water: Evidence for Plasma Formation during Multibubble Cavitation. Chem. Eur. J. 16, 11801-11803 (2010).
- Ndiaye, A. A., Pflieger, R., Siboulet, B., Molina, J., Dufreche, J. -. F., Nikitenko, S. I. Nonequilibrium Vibrational Excitation of OH Radicals Generated during Multibubble Cavitation in Water. J. Phys. Chem. A. 116, 4860-4867 (2012).
- Ndiaye, A. A., Pflieger, R., Siboulet, B., Nikitenko, S. I. The Origin of Isotope Effects in Sonoluminescence Spectra of Heavy and Light. Angew. Chem. Int. Ed. 52, 2478-2481 (2013).
- Pflieger, R., Cousin, V., Barré, N., Moisy, P., Nikitenko, S. I. Sonoluminescence of Uranyl Ions in Aqueous Solutions. Chem. Eur. J. 18, 410-414 (2012).
- Chave, T., Navarro, N. M., Nitsche, S., Nikitenko, S. I. Mechanism of Pt(IV) Sonochemical Reduction in Formic Acid Media and Pure Water. Chem. Eur. J. 18, 3879-3885 (2010).
- Thompson, L. H., Doraiswamy, L. K. Sonochemistry: science and engineering. Ind. Eng. Chem. Res. 38, 1215-1249 (2012).
- Nikitenko, S. I., Venault, L., Pflieger, R., Chave, T., Bisel, I., Moisy, P. Potential applications of sonochemistry in spent nuclear fuel reprocessing: a short review. Ultrason. Sonochem. 17, 1033-1040 (2010).
- Chave, T., Den Auwer, C., Moisy, P., Nikitenko, S. I. Sonochemical formation of Pu(IV) colloids. ATALANTE 2012 Nuclear chemistry for sustainable fuel cycles. , (2012).
- Baird, C. P., Kemp, T. J. Luminescence spectroscopy, lifetimes and quenching mechanisms of excited states of uranyl and other actinide ions. Prog. React. Kinet. 22 (2), 87-139 (1997).
- Marcantonatos, M. D. Photochemistry and exciplex of the uranyl ion in aqueous solution. J. Chem. Soc. Faraday Trans. 76, 1093-1097 (1980).
- Burrows, H. D., Kemp, T. J. Photochemistry of uranyl ion. Chem. Soc. Rev. 3, 139-165 (1974).
- Kazakov, V. P., Sharipov, G. L., Sadykov, P. A. Specific quenching of the radioluminescence from UO22+ ions by the products of radiolysis in acidic solutions. High Energy Chemistry (Khimiya Vysokikh Energii. 16, 376-377 (1982).
- Katz, J. J., Seaborg, G. T., Morss, L. R. 2nd ed. The Chemistry of the Actinide Elements. , (1986).
- Rabinowitch, E., Belford, R. L. . Spectroscopy and Photochemistry of Uranyl Compounds. , (1964).
- Mizukoshi, Y., Takagi, E., Okuno, H., Oshima, R., Maeda, Y., Nagata, Y. Preparation of platinum nanoparticles by sonochemical reduction of the Pt(IV) ions: role of surfactants. Ultrason. Sonochem. 8, 1-6 (2001).
- Fischer, C. H., Hart, E. J., Henglein, A. Ultrasonic Irradiation of Water in the Presence of 18,18O2: Isotope Exchange and Isotopic Distribution of H2O2. J. Phys. Chem. 90, 1954-1956 (1986).
- Nikitenko, S. I., Martinez, P., Chave, T., Billy, I. Sonochemical Disproportionation of Carbon Monoxide in Water: Evidence for Treanor Effect during Multibubble Cavitation. Angew. Chem. Int. Ed. 48, 9529-9532 (2009).
- Surendran, G., et al. From self-assembly of platinum nanoparticles to nanostructured materials. Small. 1, 964-967 (2005).
- Chave, T., Grunenwald, A., Ayral, A., Lacroix-Desmazes, P., Nikitenko, S. I. Sonochemical deposition of platinum nanoparticles on polymer beads and their transfer on the pore surface of a silica matrix. J. Colloid Interface Sci. 395, 81-84 (2013).
- Virot, M., et al. Catalytic dissolution of ceria under mild conditions. J. Mater. Chem. 22, 14734-14740 (2012).
- Virot, M., Chave, T., Nikitenko, S. I., Shchukin, D. G., Zemb, T., Moehwald, H. Acoustic cavitation at the water-glass interface. J. Phys. Chem. C. 114, 13083-13091 (2010).
- Virot, M., Pflieger, R., Skorb, E. V., Ravaux, J., Zemb, T., Mohwald, H. Crystalline silicon under acoustic cavitation: from mechanoluminescence to amorphization. J. Phys. Chem. C. 116, 15493-15499 (2012).
- Walther, C., et al. New insights in the formation processes of Pu(IV) colloids. Radiochim. Acta. 97, 199-207 (2009).
- Young, F. R. . Sonoluminescence. , (2004).
- Pflieger, R., Schneider, J., Siboulet, B., Möhwald, H., Nikitenko, S. I. Luminescence of trivalent lanthanide ions excited by single-bubble and multibubble cavitations. J. Phys. Chem. B. 117, 2979-2984 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone