Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Sequence-specific Labeling of Nucleic Acids and Proteins with Methyltransferases and Cofactor Analogues
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
DNA and proteins are sequence-specifically labeled with affinity or fluorescent reporter groups using DNA or protein methyltransferases and synthetic cofactor analogues. Depending on the cofactor specificity of the enzymes, aziridine or double activated cofactor analogues are employed for one- or two-step labeling.
Streszczenie
S-Adenosyl-l-methionine (AdoMet or SAM)-dependent methyltransferases (MTase) catalyze the transfer of the activated methyl group from AdoMet to specific positions in DNA, RNA, proteins and small biomolecules. This natural methylation reaction can be expanded to a wide variety of alkylation reactions using synthetic cofactor analogues. Replacement of the reactive sulfonium center of AdoMet with an aziridine ring leads to cofactors which can be coupled with DNA by various DNA MTases. These aziridine cofactors can be equipped with reporter groups at different positions of the adenine moiety and used for Sequence-specific Methyltransferase-Induced Labeling of DNA (SMILing DNA). As a typical example we give a protocol for biotinylation of pBR322 plasmid DNA at the 5’-ATCGAT-3’ sequence with the DNA MTase M.BseCI and the aziridine cofactor 6BAz in one step. Extension of the activated methyl group with unsaturated alkyl groups results in another class of AdoMet analogues which are used for methyltransferase-directed Transfer of Activated Groups (mTAG). Since the extended side chains are activated by the sulfonium center and the unsaturated bond, these cofactors are called double-activated AdoMet analogues. These analogues not only function as cofactors for DNA MTases, like the aziridine cofactors, but also for RNA, protein and small molecule MTases. They are typically used for enzymatic modification of MTase substrates with unique functional groups which are labeled with reporter groups in a second chemical step. This is exemplified in a protocol for fluorescence labeling of histone H3 protein. A small propargyl group is transferred from the cofactor analogue SeAdoYn to the protein by the histone H3 lysine 4 (H3K4) MTase Set7/9 followed by click labeling of the alkynylated histone H3 with TAMRA azide. MTase-mediated labeling with cofactor analogues is an enabling technology for many exciting applications including identification and functional study of MTase substrates as well as DNA genotyping and methylation detection.
Wprowadzenie
Specific labeling of nucleic acids1,2 and proteins3,4 is of major interest for functional characterizations, medical diagnosis and (nano)biotechnology. Here we present an enzymatic labeling method for these biopolymers which is based on S-adenosyl-l-methionine (AdoMet or SAM)-dependent methyltransferases (MTases). This class of enzymes (EC 2.1.1.) targets individual nucleophilic positions (nitrogen, oxygen, sulfur and carbon atoms) within specific residues of nucleic acids and proteins and naturally transfers the activated methyl group of the cofactor AdoMet (Figure 1A)5. In addition, MTases can utilize synthetic cofactor analogues for specific labeling with affinity tags, fluorophores or other labels (Figure 1B)6. Two classes of AdoMet analogues have been developed: Aziridine cofactors for Sequence-specific Methyltransferase-Induced Labeling (SMILing)7 and double activated AdoMet analogues for methyltransferase-directed Transfer of Activated Groups (mTAG)8.

Figure 1: Reactions catalyzed by methyltransferases (MTases). A. Methyl group transfer from the natural cofactor AdoMet (SAM) to various substrates including DNA, RNA, proteins and small biomolecules. B. Labeling/functionalization of nucleic acids and proteins (NNNNN = base pairs for DNA, nucleotides for RNA and amino acids for proteins; XXXXX = recognition sequence of the MTase with target residue in green) with synthetic cofactor analogues. Aziridine cofactors containing a reporter group (blue sphere) attached to the adenine ring are sequence specifically coupled with the target residue (left) and double-activated AdoMet analogues lead to transfer of extended alkyl chains carrying a chemical reporter Y (right) which can be labeled by bioorthogonal click reaction in a second step. Please click here to view a larger version of this figure.
Aziridine cofactors work best with DNA MTases. They contain a three membered ring with a nitrogen atom9 (or an N-mustard10,11) instead of the sulfonium center as reactive group. Protonation of this nitrogen atom activates the aziridine ring for nucleophilic attack by the target nucleotide which leads to covalent coupling of the whole cofactor with DNA. By attaching reporter groups to the adenine ring the aziridine cofactors can be used in combination with DNA MTases to label DNA in one step (Figure 1B, left)7,12. This is demonstrated in detail for the biotinylation of DNA with 6BAz13–15 (aziridine cofactor with biotin attached to the 6 position of the adenine ring) and the adenine-specific DNA MTase from Bacillus stearothermophilus (M.BseCI)16 (Figure 2, see protocol section 2: One-step labeling of DNA via aziridine cofactors). In addition to M.BseCI (5’-ATCGAT-3’ recognition sequence), the DNA MTases from Thermus aquaticus (M.TaqI, 5’-TCGA-3’), from Haemophilus heamolyticus (M.HhaI, 5’-GCGC-3’) and from Spiroplasma (M.SssI, 5’-CG-3’) have been successfully used to biotinylate DNA with 6BAz17. Furthermore, aziridine cofactors can be employed for one-step fluorescence DNA labeling18,19.

Figure 2: Sequence specific one-step biotinylation of DNA with M.BseCI and 6BAz. The DNA MTase M.BseCI recognizes the double-stranded DNA sequence 5’-ATCGAT-3’ and naturally methylates the amino group of the second adenine residue (green) using AdoMet. With the aziridine cofactor 6BAz the course of the reaction is changed and M.BseCI leads to sequence specific DNA biotinylation by coupling the whole cofactor including biotin (blue) with the target adenine. Please click here to view a larger version of this figure.
Double activated AdoMet analogues contain extended unsaturated side chains instead of a methyl group at the sulfonium center (Figure 1B, right)20. The unsaturated double or triple bond in β-position to the sulfonium center electronically compensates unfavorable steric effects within the transition state by conjugative stabilization. Since both the sulfonium center and the unsaturated bond activate the side chain for enzymatic transfer, these cofactors were named double-activated AdoMet analogues. Typically, they are used to transfer side chains with unique chemical groups (chemical reporters), like amino, alkyne and azide groups, for chemo-selective labeling in a second step8,21. In general, double-activated AdoMet analogues can not only function as cofactors for DNA MTases8,20,21 but also for RNA MTases22,23 and protein MTases24–28 allowing additional labeling of RNA and proteins. However, the extended side chains are sterically more demanding than a methyl group and enlarging the MTase active sites by protein engineering is often required to obtain efficient transfer rates. Another solution to this problem is to use an AdoMet analogue with a small propargyl group (three carbons) where the terminal alkyne serves two functions: 1. Stabilization of the transition state during enzymatic transfer and 2. reactive handle for following chemical modifications by copper-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry. It turned out that the resulting propargylic AdoMet analogue29 is quite unstable under neutral or slightly basic conditions and only of limited use. This drawback can be fixed by replacing the sulfur atom with selenium. The resulting cofactor 5‘-[(Se)[(3S)-3-amino-3-carboxypropyl]prop-2-ynylselenonio]-5‘-deoxyadenosine (SeAdoYn, Figure 3) is accepted by wild-type DNA, RNA and protein MTases30–32 which abrogate the need for protein engineering in many cases. This is exemplified by fluorescence protein labeling with the histone H3 lysine 4 (H3K4) MTase Set7/933 (Figure 3, see protocol section 3: Two-step protein labeling via double activated cofactors).
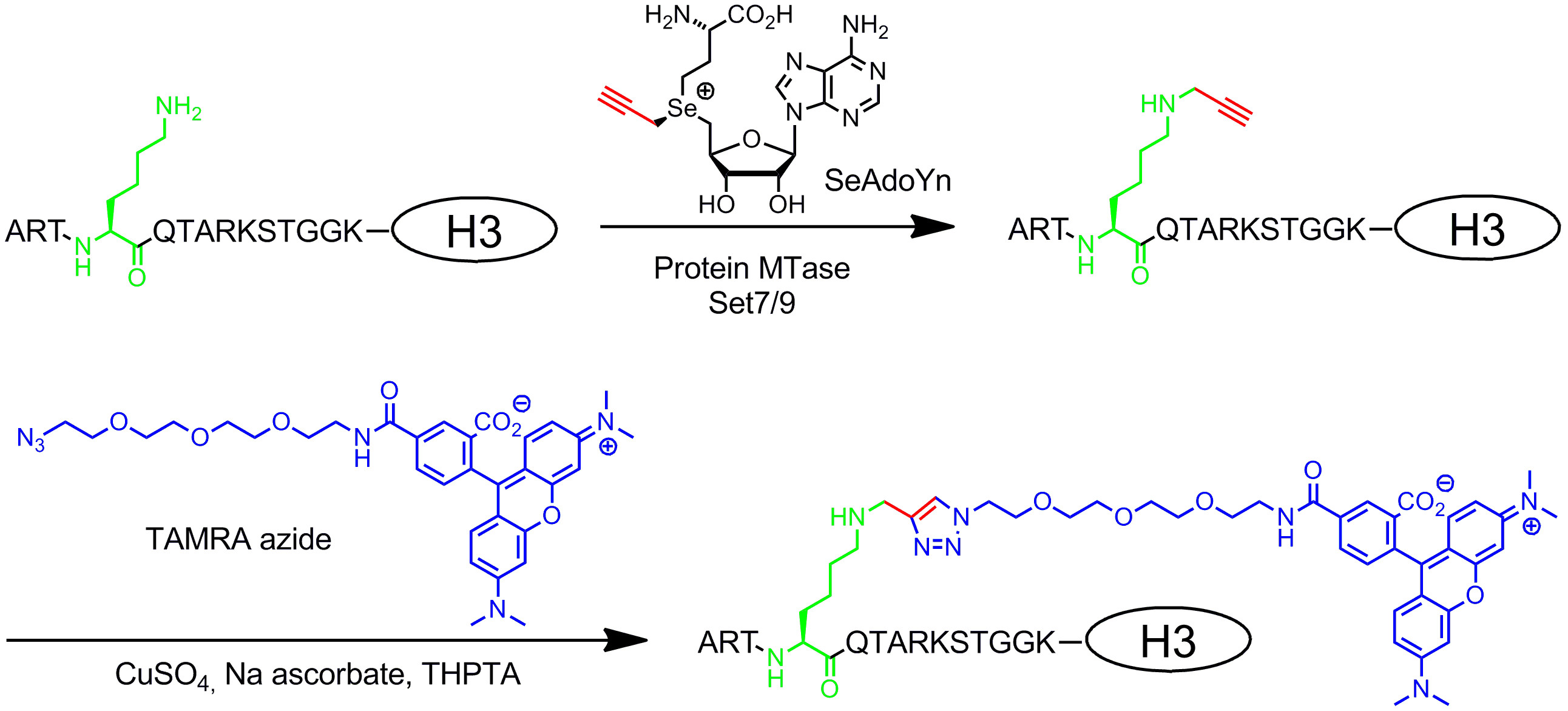
Figure 3: Sequence-specific two-step fluorescence labeling of histone H3 with Set7/9, SeAdoYn and TAMRA azide. The protein MTase Set7/9 naturally methylates the amino group of lysine 4 in histone H3 (H3K4, green) using AdoMet. With the double-activated cofactor SeAdoYn the MTase transfers a small propargyl group (red) to the lysine residue. The attached terminal triple bond is then selectively modified in a bioorthogonal click reaction (copper-catalyzed azide-alkyne cycloaddition, CuAAC) with azide-derivatized TAMRA (tetramethylrhodamine, blue) fluorophore. Please click here to view a larger version of this figure.
Protokół
1. General Instructions
- Store aziridine cofactor 6BAz (in DMSO) and protein MTase Set7/9 at -80 °C and all other reagents including double-activated cofactor SeAdoYn and DNA MTase M.BseCI (in 50% glycerol) at -20 °C.
- Determine the concentration of 6BAz and SeAdoYn via UV/Vis spectroscopy using the extinction coefficients ε269nm (6BAz) = 16,000 cm-1 M-1 and ε260nm (SeAdoYn) = 15,400 cm-1 M-1 in deionized water. Determine the concentration of MTases by the Bradford assay or, if the extinction coefficient is available, via direct absorption at 280 nm.
- Try to avoid creating bubbles by intensive pipetting or vortexing to prevent loss of enzyme activity. Instead, mix by gently pipetting up and down.
- When adding aziridine cofactors from stock solutions in DMSO make sure that final DMSO concentration in the assay is less than 5%. Always include 10 mM magnesium ions in the assay buffer to prevent non-specific reactions with DNA.
- When adding double activated cofactors from acidic stock solutions use small volumes (highly concentrated stock solutions) to avoid pH changes and make sure that the pH of the assay solution does not change significantly. Avoid thiols, e.g., β-mercaptoethanol or dithiothreitol (DTT), in the assay buffer because they can interfere with the click reaction by complexation of the required copper ions.
2. One-step Labeling of DNA via Aziridine Cofactors
- Sequence-specific Methyltransferase-Induced Label ing (SMILing) of plasmid DNA with M.BseCI DNA MTase and aziridine cofactor 6BAz.
- Thaw the cofactor solution at 20 °C and prepare the reaction mixtures on ice.
- In addition to the assay perform a “cofactor” control, to visualize any non specific modifications, and an “ enzyme” control, to make sure that the MTase preparation is free of the natural cofactor AdoMet.
- For the assay mix 2 µl of 10x modification buffer (containing 100 mM Tris-HCl, 100 mM MgCl2, 20 mM β-mercaptoethanol, pH 7.4), 2 µl of pBR322 (0.5 µg/µl), 10 eq. M.BseCI per recognition sequence on the DNA (1 recognition sequence in pBR322) and the aziridine cofactor 6BAz to a final concentration of 60 µM within a total volume of 20 µl. Add cofactor and DNA MTase last.
NOTE: β-Mercaptoethanol is toxic, corrosive and environmentally damaging. - For the “cofactor” control add deionized water instead of M.BseCI and for the “enzyme” control add deionized water instead of 6BAz.
- Mix the solutions by gently pipetting up and down.
- Incubate the tubes at 55 °C for 1 hr.
- Centrifuge briefly to collect all liquid at the bottom of the tubes.
- Restriction-modification assay to verify DNA modification.
- Prepare a solution by mixing 10 µl 10x R.TaqI buffer (containing 100 mM Tris-HCl, 50 mM MgCl2, 1 M NaCl, 1 mg/ml bovine serum albumin, pH 8.0), 80 µl deionized water and 3.3 µl of the restriction endonuclease (REase) from Thermus aquaticus (R.TaqI, 10 U/µl). Make sure to add the REase in the last step.
- To each tube from 2.1.7 add 2 µl of 10x R. TaqI buffer and 28 µl of the solution from above (2.2.1).
- Mix the solutions by gently pipetting up and down.
- Incubate the tubes at 65 °C for 30 min.
- Centrifuge briefly to collect all liquid at the bottom of the tubes.
- Electromobility shift assay (EMSA) with streptavidin to verify functional modification.
- Remove 25 µl from each tube (2.2.5) and add 2.4 µl of a streptavidin solution (1 mM with respect to streptavidin monomer in streptavidin buffer containing 100 mM Na2HPO4, 100 mM NaCl, pH 7.5; 4 equivalents of total biotin). Add 2.4 µl of streptavidin buffer to the remaining tubes.
- Incubate all tubes at 37 °C for 1 hr.
- Analysis via agarose gel electrophoresis.
- Add 5 µl of 6x loading buffer (0.25% bromophenol blue, 30% glycerol) to each tube.
- Mix the solutions gently.
- Load 10 µl of each sample into the wells of an agarose gel (1% agarose in 0.5x TBE buffer containing 1x GelRed from a 10,000x stock solution).
- Run the gel in 0.5x TBE buffer with 80 V for approx. 1 hr.
- Visualize DNA bands on a UV table (312 nm) with a CCD camera equipped with a filter (540 ± 50 nm).
NOTE: UV light is damaging to eyes and skin.
3. Two-step Protein Labeling via Double Activated Cofactors
- Methyltransferase-Directed Transfer of Activated Groups (mTAG) with Set7/9 and double-activated cofactor SeAdoYn for histone H3 lysine 4 labeling (modification step).
- Thaw the components and prepare the reaction mixtures on ice. NOTE: Always keep SeAdoYn cooled to avoid degradation.
- In addition to the assay perform a “cofactor” control, to visualize any non specific modifications, and an “enzyme” control, to exclude non-specific reactions of the fluorescent probe.
- Prepare an assay solution (20 µl) containing modification buffer (50 mM Tris-HCl, 5% glycerol, pH 8.5), 10 µM histone H3, 10 µM Set7/9 and 600 µM SeAdoYn (mixture of both epimers at selenium). In the last steps add cofactor and then MTase.
- For the “cofactor” control prepare an assay solution as in 3.1.3 and add 60 mM AdoMet to compete with the synthetic cofactor. For the “enzyme” control add deionized water instead of SeAdoYn.
- Mix the solutions by slowly pipetting up and down. Check the pH by adding 1 µl of each solution to the upper field of a pH strip (pH range 5 - 10).
- Incubate at 37 °C for 2 hr.
- In the meanwhile prepare a 12% SDS polyacrylamide gel (running gel: 357 mM Bis-Tris pH 6.5-6.8, 0.1% (w/v) APS, 0.04% (v/v) TEMED and 12% acrylamide/bisacrylamide 37.5:1; loading gel: 357 mM Bis-Tris pH 6.5-6.8, 0.1% (w/v) APS, 0.04% (v/v) TEMED and 5% acrylamide/bisacrylamide 37.5:1).
NOTE: Acrylamide/bisacrylamide is toxic and health hazardous. Wear gloves during this procedure.
- Chemical labeling of alkinylated lysine 4 in histone H3 via copper-catalyzed azide-alkyne cycloaddition (CuAAC) (labeling step).
- Just before the end of the modification reaction prepare a 5x click mix containing 3 mM CuSO4, 3 mM tris(3-hydroxypropyl-triazolylmethyl)amine (THPTA), 250 mM sodium ascorbate and 6 mM TAMRA azide with a total volume of 20 µl.
- Add 5 µl of the freshly prepared 5x click mix to each tube to start the CuAAC and quench the modification reaction.
- Mix gently by pipetting up and down.
- Protect all tubes with aluminum foil from light to avoid photo-bleaching of the fluorophore.
- Incubate at 20 °C for 1 hr.
- Protein precipitation to remove excess of free TAMRA fluorophore.
- To avoid outshining of the fluorescent labeled histone H3 by intensive in-gel fluorescence of free TAMRA fluorophore, remove excess fluorophore by precipitation of proteins (3.3.2 – 3.3.4)34.
- Add 75 µl methanol, 18.8 µl chloroform and 50 µl deionized water to each tube and vortex briefly after each addition. Centrifuge at 16,000 x g for 5 min. Remove the upper phase without disturbing the interface layer, which contains the protein.
- Add 56.3 µl methanol to the remaining phase in each tube, vortex and centrifuge at 16,000 x g for 5 min to pellet the protein. Remove the supernatant. Repeat this step to wash the pellet.
- Cover the open tubes with a lint free tissue and let them dry for 15 – 30 min.
- Analysis via SDS PAGE.
- Dissolve the precipitated proteins from 3.3.4 in 20 µl SDS loading buffer (50 mM Tris-HCl, 2.5% (w/v) SDS, 10% (v/v) glycerol, 320 mM β-mercaptoethanol and 0.05% (w/v) bromophenol blue, pH 6.8). Make sure to completely dissolve the pellet by rinsing the walls of the tubes with a pipette.
- Incubate the samples at 95 °C for 10 min and let them cool down to 20 °C.
- Centrifuge briefly to collect all liquid at the bottom of the tubes.
- Load the whole amount of each sample into the wells of an SDS polyacrylamide gel (3.1.7). Use 50 mM MOPS, 50 mM Tris-X (Tris-base), 5 mM EDTA, 0.1% (w/v) SDS as running buffer for electrophoresis.
- Run the gel with 120 V for approx. 90 min.
- Visualize the in-gel fluorescence on a UV table (312 nm) with a CCD camera equipped with a filter (540 nm ± 50 nm).
NOTE: UV light is damaging to eyes and skin.
Wyniki
One-step Labeling of DNA via Aziridine Cofactors
This example reaction is carried out with the DNA MTase M.BseCI, which modifies the second adenine residue within the double-stranded 5’-ATCGAT-3’ sequence and has one recognition site on the pBR322 plasmid (Figure 4A). To test plasmid labeling, pBR322 is challenged with the restriction endonuclease (REase) R.TaqI (5‘-TCGA-3‘). R.TaqI has seven sites on pBR322, one of which is in...
Dyskusje
One-step labeling of DNA with DNA MTases and aziridine cofactors (SMILing DNA) is a robust method but some aspects should be considered when planning the experiment.
Aziridine cofactor: The 6BAz concentration for DNA labeling with M.BseCI was 60 µM. When using other DNA MTases the cofactor concentration should be optimized, e.g. concentrations as low as 20 µM have been employed with the DNA MTase M.TaqI19. Low 6BAz concentrations have the advantage that a...
Ujawnienia
The authors disclose the following competing financial interest: E.W. is inventor on related patents.
Podziękowania
The authors thank Kerstin Glensk for preparing the MTases M.BseCI and Set7/9 and gratefully acknowledge funding by the Excellence Initiative of the German Federal and State Governments and RWTH Aachen University. The authors are happy to provide 6BAz and SeAdoYn or other cofactor analogues for collaborative research.
Materiały
| Name | Company | Catalog Number | Comments |
| 6BAz | Synthesized according to Weinhold et al., Patent number US 8,129,106, published March 6, 2012. | ||
| β-Mercaptoethanol | Serva | 28625 | |
| Acetic acid | Fisher Scientific | 10304980 | |
| Acrylamide/Bis Solution, 37.5:1 | Serva | 10688 | |
| UltraPure Agarose | Invitrogen | 16500100 | |
| Ammonium persulfate (APS) | Serva | 13375 | |
| Bis-Tris | Gerbu | 1304 | |
| Boric acid | Gerbu | 1115 | |
| Bromophenol blue Na salt | Serva | 15375 | |
| Copper(II) sulfate | Aldrich | C1297 | |
| Chloroform | Fisher Scientific | 10020090 | |
| Coomassie Brilliant Blue | Serva | 17525 | |
| EDTA disodium salt | Gerbu | 1034 | |
| Ethanol | Merck | 100983 | |
| GelRed (10,000x in water) | Biotium | 41003 | |
| Glycerol (99.5%) | Gerbu | 2006 | |
| FastRuler Low Range DNA Ladder | Thermo Scientific | SM1103 | |
| Histone H3 | Expression plasmid obtained from Dr. Philipp Voigt and Prof. Danny Reinberg; expression and isolation according to T. J. Richmond et al., J. Mol. Biol. 1997, 272, 301-311. | ||
| M.BseCI | Expression plasmid obtained from Dr. Michael Kokkinidis; expression and isolation according to Kapetaniou et al., Acta Cryst. 2006, F63, 12-14. | ||
| Methanol | Fisher Scientific | 10675112 | |
| Magnesiumchloride hexahydrate | J.T. Baker | 4003 | |
| MOPS | Gerbu | 1081 | |
| Sodium chloride | Gerbu | 1112 | |
| pH strip (Neutralit) | Merck | 1,095,330,001 | |
| pBR322 | Thermo Scientific | SD0041 | |
| R.TaqI (10 u/µl) | Thermo Scientific | ER0671 | |
| SeAdoYn | Synthesized according to Willnow et al., ChemBioChem 2012, 13, 1167-1173. | ||
| Set7/9 | Expression plasmid obtained from Prof. Danny Reinberg, expression and isolation according to D. Reinberg et al., Genes Dev.2002, 16, 479-489. | ||
| Streptavidin | Gerbu | 3058 | |
| (+)-Sodium L-ascorbate | Sigma Life Science | A7631 | |
| SDS Granular | Gerbu | 1833 | |
| di-Sodium hydrogenphosphate | Merck | 106,586 | |
| TAMRA azide | Synthesized according to reference 30: Willnow et al., ChemBioChem 2012, 13, 1167-1173. | ||
| TaqI buffer (10x) | Thermo Scientific | B28 | |
| N,N,N',N'-Tetramethylethylenediamine (TEMED) | Acros Organics | 42058 | |
| Tris-HCl | Gerbu | 1028 | |
| Tris-X (TRIS-base) | Gerbu | 1018 | |
| Tris(3-hydroxypropyltriazolyl-methyl)amine (THPTA) | Sigma-Aldrich | 762342 |
Odniesienia
- Gottfried, A., Weinhold, E. Sequence-specific covalent labelling of DNA. Biochem. Soc. Trans. 39, 623-628 (2011).
- Zohar, H., Muller, S. J. Labeling DNA for single-molecule experiments: methods of labeling internal specific sequences on double-stranded DNA. Nanoscale. 3, 3027-3039 (2011).
- Hinner, M. J., Johnsson, K. How to obtain labeled proteins and what to do with them. Curr. Opin. Biotechnol. 21, 766-776 (2010).
- Wua, Y. -. W., Goody, R. S. Probing protein function by chemical modification. J. Pept. Sci. 16, 514-523 (2010).
- Struck, A. -. W., Thompson, M. L., Wong, L. S., Micklefield, J. S-Adenosyl-methionine-dependent methyltransferases: Highly versatile enzymes in biocatalysis, biosynthesis and other biotechnological applications. ChemBioChem. 13, 2642-2655 (2012).
- Klimasauskas, S., Weinhold, E. A new tool for biotechnology: AdoMet-dependent methyltransferases. Trends Biotechnol. 25, 99-104 (2007).
- Pljevaljcic, G., Schmidt, F., Weinhold, E. Sequence-specific Methyltransferase-Induced Labeling of DNA (SMILing DNA). ChemBioChem. 5, 265-269 (2004).
- Lukinavicius, G., Lapiene, V., Stasevskij, Z., Dalhoff, C., Weinhold, E., Klimasauskas, S. Targeted labeling of DNA by methyltransferase-directed Transfer of Activated Groups (mTAG). J. Am. Chem. Soc. 129, 2758-2759 (1021).
- Pignot, M., Siethoff, C., Linscheid, M., Weinhold, E. Coupling of a nucleoside with DNA by a methyltransferase. Angew. Chem. Int. Ed. 37, 2888-2891 (1998).
- Weller, R. L., Rajski, S. R. Design, synthesis, and preliminary biological evaluation of a DNA methyltransferase-directed alkylating agent. ChemBioChem. 7, 243-245 (2006).
- Du, Y., Hendrick, C. E., Frye, K. S., Comstock, L. R. Fluorescent DNA Labeling by N-Mustard Analogues of S-adenosyl-l-methionine. ChemBioChem. 13, 2225-2233 (2012).
- Pljevaljcic, G., Schmidt, F., Scheidig, A. J., Lurz, R., Weinhold, E. Quantitative labeling of long plasmid DNA with nanometer precision. ChemBioChem. 8, 1516-1519 (1002).
- Wilkinson, S., et al. Molecular scale architecture: engineered three- and four-way junctions. Bioconjugate Chem. 19, 470-475 (2008).
- Braun, G., et al. Enzyme-directed positioning of nanoparticles on large DNA templates. Bioconjugate Chem. 19, 476-479 (2008).
- Kim, S., et al. Enzymatically incorporated genomic tags for optical mapping of DNA binding proteins. Chem. Int. Ed. 51, 3578-3581 (2012).
- Rina, M., Bouriotis, V. Cloning purification and characterization of the BseCI DNA methyltransferase from Bacillus stearothermophilus. Gene. 133, 91-94 (1993).
- Weinhold, E., Meier, T., Düfel, H., Markert-Hahn, C., Schmuck, R. Sequence-specific detection of methylation in biomolecules. US Patent. , (2012).
- Pljevaljcic, G., Pignot, M., Weinhold, E. Design of a new fluorescent cofactor for DNA methyltransferases and sequence-specific labeling of DNA. J. Am. Chem. Soc. 125, 3492-3410 (2003).
- Schmidt, F. H. -. G., Hüben, M., Gider, B., Renault, F., Teulade-Fichou, M. -. P., Weinhold, E. Sequence-specific Methyltransferase-Induced Labelling (SMILing) of plasmid DNA for studying cell transfection. Bioorg. Med. Chem. 16, 40-48 (2008).
- Dalhoff, C., Lukinavicius, G., Klimasauskas, S., Weinhold, E. Direct transfer of extended groups from synthetic cofactors by DNA methyltransferases. Nat. Chem. Biol. 2, 31-32 (2006).
- Lukinavicius, G., Tomkuviene, M., Masevicius, V., Klimasauskas, S. Enhanced chemical stability of AdoMet analogues for improved methyltransferase-directed labeling of DNA. ACS Chem. Biol. 8, 1134-1139 (2013).
- Motorin, Y., et al. Expanding the chemical scope of RNA:methyltransferases to site-specific alkynylation of RNA for click labeling. Nucleic Acids Res. 39, 1943-1952 (1943).
- Schulz, D., Holstein, J. M., Rentmeister, A. A chemo-enzymatic approach for site-specific modification of the RNA cap. Angew. Chem. Int. Ed. 52, 7874-7878 (2013).
- Peters, W., et al. Enzymatic site-specific functionalization of protein methyltransferase substrates with alkynes for click labeling. Angew. Chem. Int. Ed. 49, 5170-5173 (2010).
- Islam, K., Zheng, W., Yu, H., Deng, H., Luo, M. Expanding cofactor repertoire of protein lysine methyltransferase for substrate labeling. ACS Chem. Biol. 6, 679-684 (2011).
- Wang, R., Zheng, W., Yu, H., Deng, H., Luo, M. Labeling substrates of protein arginine methyltransferase with engineered enzymes and matched S-adenosyl-l-methionine analogues. J. Am. Chem. Soc. 133, 7648-7651 (2011).
- Islam, K., et al. Bioorthogonal profiling of protein methylation using azido derivative of S-adenosyl-l-methionine. J. Am. Chem. Soc. 134, 5909-5915 (2012).
- Islam, K., et al. Defining efficient enzyme-cofactor pairs for bioorthogonal profiling of protein methylation. Proc. Natl. Acad. Sci. U.S.A. 110, 16778-16783 (2013).
- Binda, O., Boyce, M., Rush, J. S., Palaniappan, K. K., Bertozzi, C. R., Gozani, O. A chemical method for labeling lysine methyltransferase substrates. ChemBioChem. 12, 330-334 (2011).
- Willnow, S., Martin, M., Lüscher, B., Weinhold, E. A selenium-based click AdoMet analogue for versatile substrate labeling with wild-type protein methyltransferases. ChemBioChem. 13, 1167-1173 (2012).
- Bothwell, I. R., et al. Se-Adenosyl-l-selenomethionine cofactor analogue as a reporter of protein methylation. J. Am. Chem. Soc. 134, 14905-14912 (2012).
- Tomkuviene, M., Clouet-d’Orval, B., Cerniauskas, I., Weinhold, E., Klimasauskas, S. Programmable sequence-specific click-labeling of RNA using archaeal box C/D RNP methyltransferases. Nucleic Acids Res. 40, 6765-6773 (2012).
- Nishioka, K., et al. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 16, 479-489 (2002).
- Clark, P. M., et al. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J. Am. Chem. Soc. 130, (2008).
- Lukinavicius, G., Lapinaite, A., Urbanaviciute, G., Gerasimaite, R., Klimasauskas, S. Engineering the DNA cytosine-5 methyltransferase reaction for sequence-specific labeling of DNA. Nucleic Acids Res. 40, 11594-11602 (2012).
- Neely, R. K., Dedecker, P., Hotta, J., Urbanaviciute, G., Klimasauskas, S., Hofkens, J. DNA fluorocode: A single molecule, optical map of DNA with nanometre resolution. Chem. Sci. 1, 453-460 (2010).
- Roberts, R. J., Vincze, T., Posfai, J., Macelis, D. REBASE-a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic Acids Res. 38, 234-236 (2010).
- Petrossian, T. C., Clarke, S. G. Uncovering the human methyltransferasome. Mol. Cell. Proteomics. 10, 1-12 (2011).
- Kriukiene, E., et al. DNA unmethylome profiling by covalent capture of CpG sites. Nat. Commun. 4, 2190 (2013).
- Wang, R., et al. Profiling genome-wide chromatin methylation with engineered posttranslation apparatus within living cells. J. Am. Chem. Soc. 135, 1048-1056 (2013).
- Zhang, C., Weller, R. L., Thorson, J. S., Rajski, S. R. Natural product diversification using a non-natural cofactor analogue of S-adenosyl-l-methionine. J. Am. Chem. Soc. 128, 2760-2761 (2006).
- Stecher, H., et al. Biocatalytic Fiedel-Crafts alkylation using non-natural cofactors. Angew. Chem. Int. Ed. 48, 9546-9548 (2009).
- Lee, B. W. K., Sun, H. G., Zang, T., Kim, B. J., Alfaro, J. F., Zhou, Z. S. Enzyme-catalyzed transfer of a ketone group from an S-adenosylmethionine analogue: A tool for the functional analysis of methyltransferases. J. Am. Chem. Soc. 132, 3642-3643 (2010).
- Winter, J. M., et al. Expanding the structural diversity of polyketides by exploring the cofactor tolerance of an inline methyltransferase domain. Org. Lett. 15, 3774-3777 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone