Method Article
Optimizing Attachment of Human Mesenchymal Stem Cells on Poly(ε-caprolactone) Electrospun Yarns
W tym Artykule
Podsumowanie
This article describes a range of set-ups for seeding human mesenchymal stem cells onto materials, in this case electrospun yarns, that do not cover the base of standard culture well plates in order to maximize and quantify the number of cells that initially attach compared to the known seeding density.
Streszczenie
Research into biomaterials and tissue engineering often includes cell-based in vitro investigations, which require initial knowledge of the starting cell number. While researchers commonly reference their seeding density this does not necessarily indicate the actual number of cells that have adhered to the material in question. This is particularly the case for materials, or scaffolds, that do not cover the base of standard cell culture well plates. This study investigates the initial attachment of human mesenchymal stem cells seeded at a known number onto electrospun poly(ε-caprolactone) yarn after 4 hr in culture. Electrospun yarns were held within several different set-ups, including bioreactor vessels rotating at 9 rpm, cell culture inserts positioned in low binding well plates and polytetrafluoroethylene (PTFE) troughs placed within petri dishes. The latter two were subjected to either static conditions or positioned on a shaker plate (30 rpm). After 4 hr incubation at 37 oC, 5% CO2, the location of seeded cells was determined by cell DNA assay. Scaffolds were removed from their containers and placed in lysis buffer. The media fraction was similarly removed and centrifuged – the supernatant discarded and pellet broken up with lysis buffer. Lysis buffer was added to each receptacle, or well, and scraped to free any cells that may be present. The cell DNA assay determined the percentage of cells present within the scaffold, media and well fractions. Cell attachment was low for all experimental set-ups, with greatest attachment (30%) for yarns held within cell culture inserts and subjected to shaking motion. This study raises awareness to the actual number of cells attaching to scaffolds irrespective of the stated cell seeding density.
Wprowadzenie
Scaffolds are routinely being developed and researched for biomaterial and tissue-engineering applications. As such, they are commonly seeded with cells and their in vitro behavior characterized via assays that determine cell proliferation and cell number, for example. For experiments such as these, it is imperative that the initial cell number is known and researchers often state the seeding concentration in terms of number of cells per ml or cm2. While this is good practice, especially for scale-up purposes, it does not account for the actual number of cells that adhere to the scaffold surface (which is also dependent on the adhesive properties of the biomaterial surface1). This is especially true for scaffolds that do not cover the entire base of the cell culture well plate as cells could fall away from the construct and, due to the often static nature of the experiment, may never come back into contact with the material of interest. Electrospun fiber yarns are a good example of a scaffold that does not cover the base of the well (Figure 1A). In this case, low binding well plates that have not been surface-treated should be used to prevent cells from attaching to the plate’s surface and hence distorting the results of any well-based assay.
Well plates are readily used for cell seeding onto scaffolds, but they are not the only method available. Rotary cell culture systems, a type of bioreactor developed by the Life Sciences Division at NASA in the late 1980’s, can similarly be used to seed scaffolds within a three-dimensional (3D) environment with simulated microgravity. This type of bioreactor remains a popular choice with researchers worldwide and has been incorporated in studies for cell signalling2,3, stem cells4,5 and tissue engineering6,7. What makes the rotary bioreactor preferable to well plates is the maintenance of a 3D environment, which helps to prevent differentiated cells from dedifferentiating, as is often the case when cultured within conventional 2D conditions8.
This paper investigates different techniques for seeding human mesenchymal stem cells on electrospun poly(ε-caprolactone) fiber yarns as fabricated in Bosworth et al.,9 in order to maximize the initial number of cells attaching to these scaffolds within a 4 hr period. For 2D culture, yarns were securely held within well plates or custom-made poly(tetrafluoroethylene) (PTFE) troughs and kept under static conditions, or shaken at 30 rpm. For 3D culture, yarns and cells were held within bioreactor vessels rotating at 9 rpm.
Protokół
1.Scaffold Fabrication and Sterilization
- Dissolve PCL in 1,1,1,3,3,3–hexafluoroisopropanol to give a 10% w/v concentration. As described in Bosworth et al.,9 electrospin the polymeric solution (parameters: 20 kV, 1 ml/hr, 20 cm) and collect aligned fibers on the edge of a rotating mandrel (600 rpm). With a scalpel remove the ribbon of collected fiber and then cut into shorter lengths - 3 cm (for troughs and rotary vessels) and 4 cm (for cell culture inserts) lengths.
- Using fine forceps submerge individual strips in distilled water and remove.
- Holding both ends between thumb and forefinger; manually twist the strip until it resembles thread.
- Briefly submerge this thread-like scaffold in distilled water and place on clean, non-fibrous card to dry.
- Once dry, place individually in clean microcentrifuge tubes and add 1 ml of 50% v/v ethanol in distilled water. Close the lids and leave for 24 hr.
Note: Perform the following steps under laminar flow: - Place microcentrifuge tubes in a laminar flow cabinet and aspirate the 50% v/v solution. Replace with 1 ml 70% v/v ethanol in distilled water, close lids and leave for 24 hr.
- Repeat for 90% and 100% v/v ethanol in distilled water (1 ml volumes).
- Wash scaffolds twice with phosphate buffered saline solution (PBS), 24 hr per wash (2 x 1 ml).
- Remove PBS and replace with 1 ml cell culture media. Note: Scaffolds are ready for use post-24 hr.
2 . Determining Scaffold Surface Area and Number of Cells
- Using a light microscope and imaging software, measure the diameter of the electrospun yarn along its length to determine a mean value.
- Assume the yarn to be a cylindrical rod and approximate the surface area using:
- Where A = Surface area, r = radius and h = length
Note: The surface area was calculated to be 18,902,800 µm2. Furthermore, it should be noted that the actual surface area will be greater than this calculation as the yarn is composed of hundreds of fine fibers, which will increase the surface area. Consequently a larger number of cells should be able to attach to the scaffold. However, this does not affect a direct comparison between test groups being made. - Determine the maximum number of cells that could attach to the exposed scaffold surface by:
Note: Using a light microscope and imaging software, the diameter of human mesenchymal stem cells was determined to be 20 µm (assuming cells are round), therefore in this case, number of cells = 60,200.
3. Scaffold Set-up – Cell Culture Inserts (Figure 1A)
- Under laminar flow, open the sterile 6-well cell culture inserts and separate the shorter rings with teeth from the wider ringed bodies.
- Take the ring with teeth pointing upwards. Drape one of the 4 cm scaffolds over the centre of the ring making sure it overlaps on both sides. Take the ringed body and position over the toothed ring and scaffold and push downwards making sure the scaffold stays in position and lies through the centre of the cell culture insert.
- Place the cell culture insert with scaffold into the well of a 6-well, low binding plate.
- Add 10 ml of culture media to the scaffold.
4. Scaffold Set-up – Trough (Figure 1B)
- Under laminar flow, place poly(tetrafluoroethylene) (PTFE) troughs into individual petri dishes and add 10 ml of culture media to the trough.
- Using forceps drape one of the 3 cm scaffolds into the trough, making sure its length lies parallel to the trough’s longer edge.
5. Scaffold Set-up – Bioreactor Vessel (Figure 1C)
- Under laminar flow, dispense 10 ml sterile PBS through the bioreactor vessel’s main port and leave for 10 min.
- Remove the PBS and replace with 8 ml culture media.
- Using forceps, insert one of the 3 cm scaffolds into the vessel via the main port.
- Close off the main port.
6. Cell Counting
- Culture human mesenchymal stem cells (hMSC) derived from bone marrow according to manufacturer protocol up to passage 4 prior to harvesting.
- Aspirate the media from a 75 cm2 flask containing hMSCs derived from bone marrow (passage 4, 80% confluency).
- Wash the cells with 10 ml sterile PBS and aspirate.
- Add 3 ml trypsin and incubate the flask at 37 °C, 5% CO2 until the cells have dislodged from the flask surface.
- Add 7 ml culture media to inactivate the enzyme and transfer this total volume (10 ml) to a centrifuge tube.
- Resuspend this cell suspension by pipetting up and down several times to homogeneously disperse cells within the media and limit cell agglomerates (10 ml pipette).
- Remove 20 µl of cell suspension and transfer to a haemocytometer.
- Place the haemocytometer under a light microscope and image at x10 objective.
- Focus the gridlines and count the number of cells in the 4 x 4 squares in each corner of the grid and which fall within the square and those that cross the right hand or bottom boundary line. Count for each set of 4 x 4 squares (4 counts per grid).
- Repeat the resuspension and cell count three times (steps 6.6-6.9).
- Calculate the average cell count and determine the volume of media required for cell resuspension (cell concentration 60,200 in 200 µl). For example, determine the average cell count from the haemocytometer and then determine the overall average number of cells from the three separate counts. Multiply this by 1 x 104 to give number of cells per ml and then multiply by the total volume of cell suspension to give total number of cells. Use the following equation to determine volume of media required for resuspension:
- Centrifuge the cell suspension at 241 x g for 5 min.
7. Cell Seeding
- Aspirate the media from the centrifuged tube leaving the cell pellet and replace with the calculated media volume.
- Resuspend the cells and media for an even mix.
- Using a P200 Gilson pipette, slowly dispense 200 µl of cell suspension onto each scaffold by running the tip of the pipette along the scaffold length and below the media liquid surface. Leave undisturbed for 20 min.
- For bioreactor vessels, dispense 200 µl of cell suspension through the main port. Top-up the remaining 2 ml culture media via the syringe ports to give a total volume of 10 ml.
8. Experimental Start
- Transfer the bioreactor vessels to the RCCS-4DQ bioreactor and set to rotate at 9 rpm.
- Transfer the well plates with cell culture inserts and troughs to the shaker plate and set to rotate at 30 rpm.
- Transfer the well plates with cell culture inserts and troughs to the shelf of a cell incubator set at 37 °C, 5% CO2 (static culture).
9. DNA Assay
- Prepare solutions – lysis buffer, 1x TE buffer, DNA standards and cell DNA working solution - as per the manufacturer instructions.
- After 4 hr, remove the samples from the incubator and place under laminar flow.
- Remove the media fractions from all samples and place in separately labelled centrifuge tubes. Centrifuge the tubes 241 x g . Remove the supernatant and add 3 ml lysis buffer. Resuspend the solution to break-up the cell pellet.
- Using forceps remove the scaffolds and place in centrifuge tubes containing 3 ml lysis buffer (for the scaffolds within the bioreactor vessels, remove the scaffold prior to aspirating the media). For scaffolds held within cell culture inserts, first free the scaffold by cutting the scaffold close to the insert’s edge using a scalpel.
- Add 3 ml of lysis buffer to each scaffold receptacle and scrape the surface (vigorously agitate for the bioreactor vessels). Remove the lysis buffer and place in separately labelled centrifuge tubes.
- Vortex each centrifuge tube for approximately 1 min to ensure sufficient agitation of the cells and buffer and to encourage lysis of the cell membrane.
- In a black 96 well plate, add 100 µl of lysis buffer for each sample fraction – scaffold, media and well (duplicate).
- In the dark, add 100 µl of cell DNA solution to all wells containing lysis buffer and mix gently.
- Include wells with lysis buffer containing no DNA and cell DNA solution to provide a negative and positive control for the well plate.
- Using a fluorescence plate-reader, measure the absorbance of the wells using 485 nm excitation and 520 nm emission.
- Compare the data with the standard curve generated from the DNA standards as per the manufacturer instructions.
10. Scanning Electron Microscope (SEM) Fixation
- Perform the following steps under laminar flow: After 4 hr, remove all scaffolds from their receptacles and place within a new 6-well plate (separate wells).
- Wash the scaffolds twice with PBS.
- Perform the following steps on the open bench: To each well, add 2 ml of 1.5% v/v glutaraldehyde in PBS to ensure complete coverage of the scaffold.
- Leave the plate for minimum 30 min at 4 °C for cell fixation.
- Remove the fixative solution and wash the scaffolds twice with PBS.
- Dehydrate the scaffolds with increasing concentrations of ethanol in distilled water starting with 50% v/v, followed by 70% v/v and 90% v/v. For each concentration, fully submerse the scaffolds in solution (2 ml) and leave for 3 min. Discard the solution and repeat.
- Dehydrate in 100% ethanol by fully submersing the scaffolds in solution (2 ml) and leaving for 5 min. Discard the solution and repeat.
- Chemically dry the scaffolds using hexamethyldisilazane (HMDS) within a fume cupboard. Immerse the scaffolds in HMDS (2 ml) and leave for 5 min. Remove the HMDS and repeat.
- Remove the HMDS and allow the scaffolds to dry. Mount the scaffolds on commercially available SEM stubs (in this case stainless steel stubs with adhesive carbon tabs).
- To ease viewing within the SEM, coat the samples using a gold-sputter coater for 2 min to ensure a thin and even coverage.
- Place samples within the SEM and visualize the cell-seeded scaffolds using a 5 KeV electron beam.
Wyniki
The results highlight the location of cells following 4 hr post-seeding for each experimental set-up investigated. Figure 2A demonstrates the percentage of cells that have attached to the scaffold surface during this time. A conversion factor of 8.5 pg/cell was used to convert the measured DNA content into cell number and thus determine the percentage of cells10. For all seeding set-ups investigated, the percentage of cell attachment is relatively low, with greatest cell adherence (30%) for scaffolds held within cell culture inserts and shaken at 30 rpm (Insert Shaker). Lowest adherence (15%) was for scaffolds held within the cell culture inserts and kept under static conditions (Insert Static).
A large number of cells were present within the media fraction (Figure 2B), most notably for cell culture inserts held within low binding plates (Insert Static) and rotary vessels being 50% and 51% respectively. Scaffolds held within the troughs demonstrated a raised number of cells present within the holder itself – 48% of cells for Trough Shaker and 50% for Trough Static.
Scanning electron microscopy allowed a visual assessment of the cell-seeded scaffolds (Figure 3). Representative images highlighted a limited presence of cells on the fibrous surface, irrespective of seeding set-up. However, a greater number of cells and cell agglomerates were present on the scaffolds held within the cell culture inserts and shaken at 30 rpm (Insert Shaker).
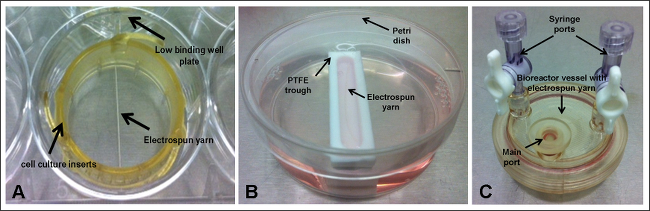
Figure 1. Experimental Set-ups, where Electrospun Yarn is Held within; (A) cell culture inserts and low binding well plate; (B) poly(tetrafluoroethylene) (PTFE) trough and petri dish; and (C) bioreactor vessel. Please click here to view a larger version of this figure.
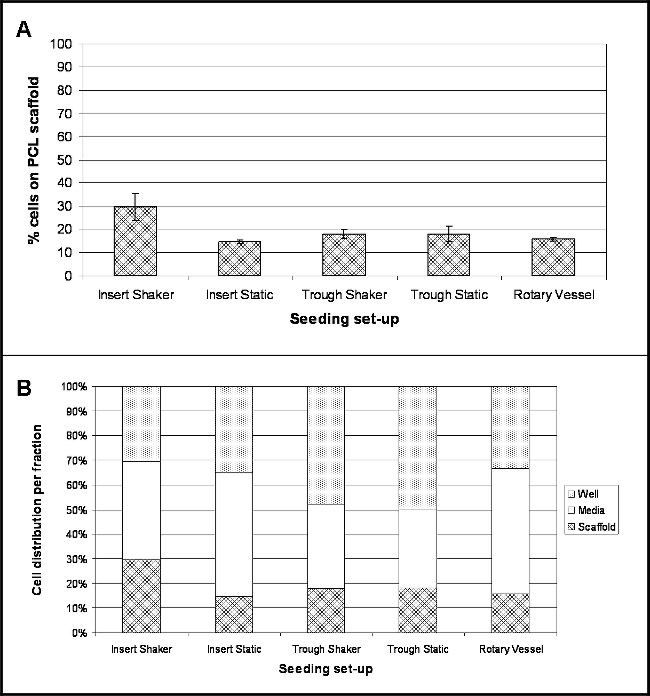
Figure 2. Location of Cells 4 hr Post-seeding onto PCL Electrospun Yarns using Different Seeding Set-ups. (A) Demonstrates the percentage of cells that have attached to the PCL scaffold (mean ± standard deviation); (B) highlights the percentage spread of cell location within the three fractions – media, well and scaffold (n = 4, data presented as mean values). Please click here to view a larger version of this figure.

Figure 3. Representative Scanning Electron Micrographs for PCL Electrospun Yarns with Human Mesenchymal Stem Cells, 4 hr after Initial Seeding using Different Experimental Set-ups. (All images at 1,000X magnification, scale bar = 130 µm.) Please click here to view a larger version of this figure.
Dyskusje
Electrospun fiber matrices fabricated from biopolymers are regularly used to support cell attachment and proliferation for biomaterial and/or tissue engineering applications11,12. In these cases, the matrices are often thin sheets of fibers that easily cover the entire base of a cell culture well plate and thus are in complete contact with seeded cells which improves cell attachment. However, if the biomaterial scaffold does not fully cover the base of the well plate, there is a high chance that a large proportion of the seeded cells will not stay in contact with the scaffold and ultimately will not be able to attach. This study investigated several different methods for seeding cells onto scaffolds that do not cover the base of the well plate, in order to determine an optimized technique that could be recommended for future cell-based experiments.
Five different set-ups were investigated (Figure 1): scaffolds (electrospun yarn) held using cell culture inserts within low binding well plates and either kept under static conditions or shaken at 30 rpm; scaffolds placed within narrow PTFE troughs and held static or shaken at 30 rpm; and scaffolds housed inside bioreactor vessels rotating at 9 rpm. Determining the number of cells that had adhered to the electrospun yarns by DNA assay demonstrated a low percentage of attachment for all seeding set-ups (Figure 2); and this was further confirmed from scanning electron micrographs (SEM) (Figure 3). Greatest cell attachment – 30% or ~18,060 cells -was observed for yarns that were held within cell culture inserts and subjected to continuous motion. Interestingly, lowest cell attachment (15%) was achieved for yarns held by cell culture inserts but kept under static conditions, which would suggest that the inclusion of radial motion has a positive effect on keeping cells in contact with the scaffold. However, it should be noted that continuous circling of the media’s flow might be responsible for the cell agglomerates observed from the SEM images. The shaker plate was set on its lowest setting – 30 rpm – which could be a limitation to this set-up. Using a slower radial motion may help to prevent or reduce cell agglomeration and could also improve cell attachment as cells will experience less force. Future experiments should focus on optimising the ideal shaker speed for improved cell attachment. Incorporating motion for yarns held within the troughs did not result in a similar trend, with both scenarios yielding 18% attachment (~10,836 cells); though this may be due to the partial floatation of the scaffolds within the troughs (observed for troughs placed on the shaker plate) as they were not anchored to the base. Partial floating of the scaffold will prevent any cells that have sunk to the bottom of the trough from coming into contact with the material and adhering. For this particular set-up, the trough was housed within a petri dish and a total 10 ml volume of media added. The small dimensions of the trough means that the majority of the media is present within the petri dish and if there is any movement, cells may drift away from the trough into the petri dish and remain completely out of reach of the scaffold. To overcome these limitations, further experiments should include an extra step in the protocol, whereby the ends of the scaffolds are pinned to the base of the troughs using sterile fine-needles, as this should prevent their floatation and movement (particularly for scaffolds exposed to radial motion), which ultimately should lead to an increased number of cells attaching to the scaffold. 16% of cells had attached to the yarns present within the rotary vessels. Despite being a well-established technique for 3D culture, problems did arise with the removal of scaffolds from the vessels’ main port, which may have resulted in loosely attached cells being lost. Vessels that can be fully opened would eliminate this problem; these are available to purchase, but are considerably more expensive than the disposable vessels used in this study.
This study demonstrates the current issues with seeding scaffolds that do not cover the entire base of standard cell culture well plates. Seeding a known cell number resulted in less than a third attaching to the scaffold, despite the scaffold’s surface area allowing for all cells to adhere. This could have detrimental consequences in other cell-based assays that may assess the biocompatibility and cell-material / cell-cell behaviour and interactions with the scaffold as a potential future medical device. Further limitations of the study may include the 4 hr time-point – despite being long enough to ensure initial cell seeding (cells have been shown to firmly attach to substrates within thirty minutes13,14,15), it may be reasonable to investigate later time-points providing cells do not proliferate during a longer time-frame as this would otherwise skew the starting cell number. Reducing the volume of media, in this case 10 ml, could also improve contact between the cells and scaffold and ultimately increase cell attachment. Future studies should also consider cell viability as the process of cell seeding can cause cell damage and/or cell death16. Cell DNA assays do not differentiate between viable and non-viable cells, as such a live/dead assay, for example, would highlight the level of viability.
This investigation raises awareness to the actual number of cells that attach to the scaffold despite seeding a known quantity. For studies that rely on the starting number of cells, it is highly important that researchers know exactly how many of that figure do in fact adhere to the substrate of interest.
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
The authors would like to thank and acknowledge the Medical Research Council for funding this research - MRC-DPFS grant code G1000788-98812.
Materiały
| Name | Company | Catalog Number | Comments |
| Distilled water | in-house supply | n/a | |
| Ethanol | Merck | 1117271000 | |
| Phosphate Buffered Saline solution | Life Technologies | 70013016 | |
| Human mesenchymal stem cells | PromoCell GmbH | C-12974 | |
| MSC culture media | PromoCell GmbH | C-28010B | Warmed to 37 oC before use |
| Supplement mix | PromoCell GmbH | C-39810 | Add to culture media |
| Antibiotic/antimyotic mix | Sigma-Aldrich | A5955 | Add to culture media |
| Trypsin (0.05%) EDTA (0.02%) | Sigma-Aldrich | 59417C | Warmed to 37 °C before use |
| Cell culture flasks (T75) | Becton Dickinson Ltd | 353110 | |
| Low binding 6-well plates | Costar Corning | 3471 | |
| 6-well CellCrowns | Scaffdex | C00003S | |
| Petri-dish 50 ml deep | Sterilin | 124 | |
| PTFE troughs | in-house production | n/a | |
| Disposable RCCS vessels 10 ml | Synthecon | D-410 | |
| 4 Vessel Rotary Cell Culture System bioreactor | Synthecon | RCCS-4DQ | |
| Shaker plate | Stuart | SSM1 | Mini Orbital Shaker |
| Haemocytometer | Digital Bio | DHC-F01 | Disposable C-Chip |
| Centrifuge tube | Deltalab | 352096, 429901 | 15 ml and 50 ml |
| Centrifuge | Hettich | Rotafix 32 A | |
| Syringe 3 ml | Shield Medicare Ltd | 3039820 | |
| Pipettes | Sterilin | 40305, 47310, 40125 | 5, 10 and 25 ml |
| Gilson pipettes | SLS | F144801, F144802, F123600, F123601, F123602 | P2 - P1000 |
| Pipette tips | SLS | PIP7852, PIP7834, PIP7840 | |
| Micro test tube 1.5 ml | Eppendorf | 30125.15 | |
| Triton X-100 | Sigma | 9002-93-1 | |
| PicoGreen Assay | Invitrogen | P7589 | Assay set-up in the dark |
| Black 96-well plate | Greiner Bio One | 655086 | |
| Fluorescent plate-reader | BGM Labtech | FLUOstar Optima | |
| Glutaraldehyde 25% | TAAB Laboratories | G002 | Made to a concentration of 1.5% v/v in PBS |
| Hexamethyldisilazane (HMDS) | Sigma | 999-97-3 | |
| Aluminium stubs (SEM) | Agar Scientific | G301 | |
| Carbon tabs (SEM) | Agar Scientific | G3347N | |
| Gold sputter coater | Edwards | S150B | |
| Scanning Electron Microscope (SEM) | Phenom World | Phenom Pro |
Odniesienia
- Jauregui, H. O. Cell adhesion to biomaterials. The role of several extracellular matrix components in the attachment of non-transformed fibroblasts and parenchymal cells. ASAIO transactions/American Society for Artificial Internal Organs. 33 (2), 66-74 (1986).
- Puca, A., Russo, G., Giordano, A. Properties of Mechano-Transduction via Simulated Microgravity and its Effects on Intracellular Trafficking of VEGFR's. Oncotarget. 3 (4), 426 (2012).
- Vincent, L., Avancena, P., Cheng, J., Rafii, S., Rabbany, S. Y. Simulated microgravity impairs leukemic cell survival through altering VEGFR-2/VEGF-A signaling pathway. Annals of biomedical engineering. 33 (10), 1405-1410 (2005).
- Rungarunlert, S., Klincumhom, N., Tharasanit, T., Techakumphu, M., Pirity, M. K., Dinnyes, A. Slow Turning Lateral Vessel Bioreactor Improves Embryoid Body Formation and Cardiogenic Differentiation of Mouse Embryonic Stem Cells. Cellular Reprogramming. 15 (5), 443-458 (2013).
- Wu, X., Li, S. H., Lou, L. M., Chen, Z. R. The Effect of the Microgravity Rotating Culture System on the Chondrogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Molecular biotechnology. 54 (2), 331-336 (2013).
- Wang, Y., et al. Rotating Microgravity-Bioreactor Cultivation Enhances the Hepatic Differentiation of Mouse Embryonic Stem Cells on Biodegradable Polymer Scaffolds. Tissue Engineering Part A. 18 (21-22), 2376-2385 (2012).
- Lv, Q., Deng, M., Ulery, B. D., Nair, L. S., Laurencin, C. T. Nano-ceramic Composite Scaffolds for Bioreactor-based Bone Engineering. Clinical Orthopaedics and Related Research. 471 (8), 2422-2433 (2013).
- Hammond, T. G., Hammond, J. M. Optimized suspension culture: the rotating-wall vessel. American Journal of Physiology-Renal Physiology. 281 (1), F12-F25 (2001).
- Bosworth, L. A., Alam, N., Wong, J. K., Downes, S. Investigation of 2D and 3D electrospun scaffolds intended for tendon repair. Journal of Materials Science: Materials in Medicine. 24 (6), 1605-1614 (2011).
- Dormer, N. H., Qiu, Y., Lydick, A. M., Allen, N. D., Mohan, N., Berkland, C. J., Detamore, M. S. Osteogenic differentiation of human bone marrow stromal cells in hydroxyapatite-loaded microsphere-based scaffolds.. Tissue Engineering Part A. 18 (7-8), 757-767 (2011).
- Rayatpisheh, S., Heath, D. E., Shakouri, A., Rujitanaroj, P. O., Chew, S. Y., Chan-Park, M. B. Combining cell sheet technology and electrospun scaffolding for engineered tubular, aligned, and contractile blood vessels. Biomaterials. 35 (9), 2713-2719 (2014).
- Wismer, N., Grad, S., Fortunato, G., Ferguson, S. J., Alini, M., Eglin, D. Biodegradable electrospun scaffolds for annulus fibrosus tissue engineering: effect of scaffold structure and composition on annulus fibrosus cells in vitro. Tissue Engineering Part A. (3-4), 672-682 (2014).
- Yavin, E., Yavin, Z. Attachment and culture of dissociated cells from rat embryo cerebral hemispheres on polylysine-coated surface. The Journal of cell biology. 62 (2), 540-546 (1974).
- Chen, H. Guan, of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proceedings of the National Academy of Sciences. 91 (21), 10148-10152 (1994).
- Grant, D. S., Tashiro, K. -. I., Segui-Real, B., Yamada, Y., Martin, G. R., Kleinman, H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 58 (5), 933-943 (1989).
- Carrier, R. L., Papadaki, M., Rupnick, M., Schoen, F. J., Bursac, N., Langer, R., Freed, L. E., Vunkaj-Novakovic, G. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnology and bioengineering. 64 (5), 580-589 (1999).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone