Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Primer for Immunohistochemistry on Cryosectioned Rat Brain Tissue: Example Staining for Microglia and Neurons
W tym Artykule
Podsumowanie
This introductory level protocol describes the reagents, equipment, and techniques required to complete immunohistochemical staining of rodent brains, using markers for microglia and neuronal elements as an example.
Streszczenie
Immunohistochemistry is a widely used technique for detecting the presence, location, and relative abundance of antigens in situ. This introductory level protocol describes the reagents, equipment, and techniques required to complete immunohistochemical staining of rodent brain tissue, using markers for microglia and neuronal elements as an example. Specifically, this paper is a step-by-step protocol for fluorescent visualization of microglia and neurons via immunohistochemistry for Iba1 and Pan-neuronal, respectively. Fluorescence double-labeling is particularly useful for the localization of multiple proteins within the same sample, providing the opportunity to accurately observe interactions between cell types, receptors, ligands, and/or the extracellular matrix in relation to one another as well as protein co-localization within a single cell. Unlike other visualization techniques, fluorescence immunohistochemistry staining intensity may decrease in the weeks to months following staining, unless appropriate precautions are taken. Despite this limitation, in many applications fluorescence double-labeling is preferred over alternatives such as 3,3'-diaminobenzidine tetrahydrochloride (DAB) or alkaline phosphatase (AP), as fluorescence is more time efficient and allows for more precise differentiation between two or more markers. The discussion includes troubleshooting tips and advice to promote success.
Wprowadzenie
Immunohistochemistry is a process for detecting antigens (i.e. proteins) in tissue sections by using primary antibodies which bind specifically to the antigens of interest. Immunohistochemistry was pioneered by J.R. Marrack in 1934 when he determined that antibodies could localize antigens with great specificity1. Beginning in 1942, some of the first in vitro studies using fluorescent antibodies to visualize immunohistochemistry were published2,3, after which the first in vivo histochemical study was published 4. During the 1960’s, three decades after the inception of immunohistochemical methods, enzyme-conjugated antibodies began to be used as secondary reagents. These methods were simultaneously and independently developed in France and the U.S.5,6. Today, a wide array of antibodies provides endless possibilities for immunohistochemistry studies7.
The overall goal of this correspondence is to provide a brief introduction into immunohistochemical staining; it is not meant to be a comprehensive and exhaustive review of this technique. In the method outlined, immunohistochemical techniques for two antigens are presented (markers for microglia and neurons) for staining of paraformaldehyde perfused, sucrose cryoprotected, cryosectioned rat brain. Immunohistochemical staining begins with blocking nonspecific antigen binding to reduce background staining. Next, incubation with primary antibody allows for binding to a specific antigen in the tissue. Following the primary antibody, another antibody, termed secondary antibody, is applied to link the primary antibody to a conjugated visualization signal8. The secondary antibody targets the immunoglobulin G (IgG) domain specific to the species in which the primary antibody was raised. The secondary antibody amplifies the signal of the primary antibody since the Fab regions of the secondary antibody bind to multiple sites on the IgG domain of the primary antibody. Either enzymes or fluorescent molecules conjugated to the Fc regions of the secondary antibody enable visualization. For example, a rabbit anti-Iba1 primary antibody is a rabbit IgG molecule specific for Iba1. When donkey anti-rabbit IgG is applied as a secondary antibody, it will recognize and bind to multiple regions of the rabbit anti-Iba1 IgG (see Figure 1). The donkey antibody can be visualized by various methods. This correspondence focuses on detection of a fluorophore conjugated to the secondary antibody, which recognizes the primary antibody, for visualization by fluorescent microscopy. In fluorescent immunohistochemistry, a nuclear stain such as Hoechst or DAPI can be used to visualize all nuclei.
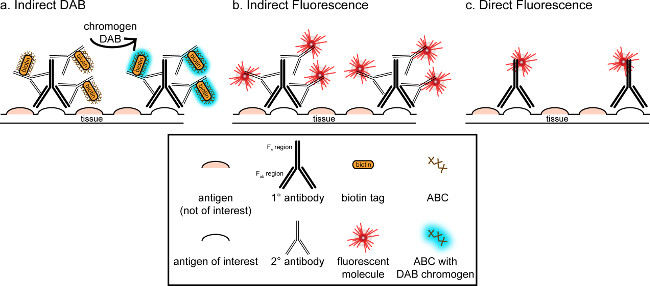
Figure 1: Schematic representation of direct vs. indirect antibody labeling techniques. Antibodies bind to the antigen of interest and can be amplified by secondary antibodies raised against the species of the primary antibodies. This technique can be performed using avidin-biotin complex (ABC) for amplification and DAB for visualization (A), or a directly conjugated fluorescent secondary antibody (B). Alternatively, primary antibodies can be directly conjugated with many different tags, including biotin or a fluorophore (C). Please click here to view a larger version of this figure.
An alternative method for visualization of immunohistochemical staining uses 3,3'-diaminobenzidine tetrahydrochloride (DAB; see Figures 1 and 2). This differs from fluorescence by using a biotinylated or horse-radish peroxidase (HRP) conjugated secondary antibody, which provides an enzyme to convert DAB to a precipitate that is visible under bright field microscopy. In instances where a single antigen is of interest or staining is required to be long lasting, DAB may be more appropriate than fluorescent staining. However, DAB staining is not well-suited for differentiation between multiple markers, especially if two nuclear antigens are of interest. For information on DAB materials and protocol modifications, consult Table 1. Alternately, nitro blue tetrazolium chloride/5-Bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) can be used to visualize an alkaline phosphatase (AP) conjugated secondary antibody.
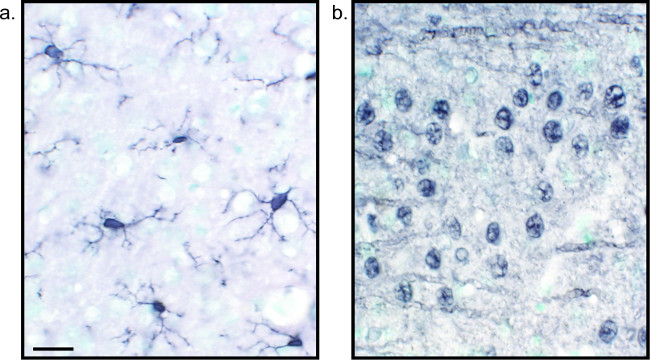
Figure 2: Representative images of nickel-enhanced DAB single-labeled rat brain tissue sections. Rat brain sections which are labeled with nickel-enhanced DAB for Iba1 (A) and Pan-neuronal (B) allow for long-lasting analysis of microglia or neurons alone. Scale bar 20 µm. Please click here to view a larger version of this figure.
One must consider the estimated abundance of the antigen of interest within the tissue being analyzed. Indirect methods (as described above) are useful for targets with low abundance. When the antigen of interest is in high abundance, direct methods can be applied. Direct methods involve a primary antibody that is directly conjugated to a visualization signal, and thus no secondary antibody is required. This method simplifies the staining process, but eliminates the amplification achieved by indirect methods. Using a directly conjugated primary antibody also eliminates cross-reactivity of secondary antibodies when double-labeling.
This communication details the protocol for double-labeling with Iba1 and Pan-neuronal (details in Table 1). Iba1 stains microglia in many activation states, including ramified, hyper-ramified, activated, amoeboid, and rod. Pan-neuronal stains neuronal axons, dendrites, and soma. Since Iba1 stains most microglia and Pan-neuronal targets the neuron, this combination of stains is useful in gaining a broad understanding of microglia-neuron interactions.
In sum, immunohistochemical staining relies on the careful selection of antibodies. As the research question becomes more specific, antibodies raised to alternate antigens may be desired. To target a specific microglial activation state, one may elect to use CD45 or CD68 antibodies, rather than Iba1. Further, in working with mice, F4/80 may provide the necessary results. Similarly, neuronal elements can be specifically targeted with antibodies raised against the nucleus, synapse (pre- or post-), axon, and growth cone. Additionally, there are other markers which differentiate the age of the neuron (Double-cortin, NeuN), and neuronal regeneration (GAP-43).
Protokół
NOTE: All procedures were carried out in compliance with The Institutional Animal Care and Use Committee (IACUC) of the University of Arizona. A list of recommended materials and equipment can be found in Table 1.
1. Tissue Preparation
- Perfusion
- Euthanize rodent with an overdose of sodium pentobarbital (25 mg/kg, IP), and perfuse transcardially with phosphate buffered saline (PBS) until completely exsanguinated (3-5 min) at a flow rate of 8 ml/min. For in-depth perfusion instructions, see Gage et al 20129.
- Immediately following PBS flush, fix tissue by perfusing with 4% paraformaldehyde in PBS for 15-20 min at a flow rate of 8 ml/min.
- Remove brain and place in 4% paraformaldehyde for 24 hr, followed by graded sucrose solutions (15%, 30%, 30%, in sequence; prepared in tris buffered saline) at 4 °C. Transfer the brain to the subsequent sucrose solution only after the brain has sunk in each solution. Note: Usually, 5 days in each solution is sufficient time for tissue to sink.
- Tissue Freezing and Cryosectioning
- Place the brain in embedding medium, such as O.C.T. compound and submerge in isopentane at a temperature of -35 °C. Allow the brain to freeze for a minimum of 10 min, and then store at -80 °C. Problems can arise if diligence to temperature is not taken; please see discussion for troubleshooting information.
- Cut serial coronal sections at a thickness of 20 µm and a temperature of -20 °C. Collect tissue onto positively charged slides. Brain sections may be placed in a slide box wrapped in foil in a zip-top bag and stored long-term at -80 °C. This method of storage creates a double boundary to prevent exposure to air and frost.
2. Tissue Processing
NOTE: Example equipment and materials required for staining are shown in Figure 3. Alternatives are available, however, these images will aid those new to immunohistochemical staining to visualize appropriate items prior to purchasing.

Figure 3: Example items required for immunohistochemical staining. The black box shown in (A) is an ideal humidity chamber for immunofluorescence, as slides are protected from light without the need to wrap the box on foil. Following sectioning, slides can be stored in a box such as the yellow box shown in (B). Wrapping the box tightly in foil and placing in a zip-top bag prior to the freezer helps protect the tissue samples from freezer burn. An example of slides is given in (C), with different staining dishes depicted in (D) and (E). Coverslips can vary in size and thickness (F), however 1.2 thick coverslips provide nice imaging results on most upright and confocal microscopes. A pencil such as that shown in (G) can be used to label slides. Permanent markers should be avoided as the ink can run, affecting both staining and the ability to determine what the sample is. A mini pap pen such as that shown in (H) enables a repellent border to be drawn on slides.
- Slide preparation
- Remove slides from freezer and thaw at room temperature.
- Optional: If sections have previously floated from slides, place thawed slides in an oven at 60 °C for no more than 4 hrs to help prevent tissue sections from floating off slides.
- Place slides in a slide rack and corresponding dish.
- Wash slides three times in PBS for 5 min each, changing solution between washes. From this step forward, avoid having sections without liquid for extended periods of time. Note: If sections dry out, background staining is increased and meaningful data cannot reliably be obtained.
- Remove slides from freezer and thaw at room temperature.
- Tissue Staining
- In a light-tight staining box, create a “humidity chamber” with lint-free tissues soaked with deionized water.
- Dry the edges of the slide with a lint-free tissue, use a mini pap pen to make a liquid repellent border at the very edge of the slide, away from tissue sections. This border should ensure ample space between the meniscus of the liquid and the edge of the tissue so that surface tension does not affect staining.
NOTE: The pap pen repellent border can be applied prior to 2.1.3 if the antibodies of interest do not require microwave antigen retrieval. If the pap pen has been applied prior to washing in PBS, the integrity of the liquid-repellent border must be checked at this step. Use a mini-pap pen to fill in any gaps in the border. - With slides laid horizontally, block nonspecific antigen binding by incubating in 4% v/v serum in PBS (block solution). Pipette 300 µl of block solution per slide for 1hr at room temperature. Make sure the block solution extends to the pap pen at the edge of the slide and completely covers the tissue to avoid uneven staining caused by surface tension near the tissue.
- Use serum from the same species in which secondary antibody is made. Note: In this procedure, the secondary antibodies are made in donkey, and thus donkey serum is used. If secondary antibodies from two or more different species are used, include serum from each species.
- Pipette primary antibody onto slides. Note: Antibody concentrations for this staining have been optimized at 1:5,000 and 1:500 for Iba1 and Pan-neuronal, respectively. These concentrations have been found to show meaningful staining with an absence of background staining.
- Dilute block solution to 1% serum in PBS and add primary antibodies. Pipette 300 µl of primary antibody solution in 1% serum per slide. Again, ensure the fluid is to the edge of the pap pen. Incubate overnight at 4 °C.
- Include three control slides: one that contains neither Iba1 nor Pan-neuronal antibodies, one with Iba1 without Pan-neuronal antibody, and one with Pan-neuronal antibody without Iba1. Stain these slides in the same run with the same solutions, however omit the primary antibodies to test the non-specific binding of the secondary antibodies.
- The following morning, wash slides three times in PBS for 5 min each, changing solution between washes.
- Fluorescent antibodies are light-sensitive, therefore, from this step forward, minimize light exposure by ensuring wash containers are wrapped in foil and hybridization boxes are either black or incubated in the dark. Pipette the appropriate secondary antibodies on all slides and incubate for 60 min at room temperature at a concentration of 1:250 in block solution (see step 2.2.3) in a light-tight “humidity chamber” (see step 2.2.1).
- Use secondary antibodies of different wavelengths. Here, for the primary antibody rabbit anti-Iba1, use donkey anti- rabbit 594 as the appropriate secondary antibody. For the primary antibody mouse anti-Pan-neuronal, use donkey anti-mouse 488 as the appropriate secondary antibody. Alternately, use anti-rabbit 488 and anti-mouse 594.
- Wash slides three times in PBS for 5 min each.
- Optional: perform nuclear staining.
- Place in Hoechst (or other nuclear stain) at a concentration of 0.03 µg/ml in double distilled H20 for exactly 60 sec.
- Wash slides three times in PBS for 5 min each.
- Wash in ddH20.
- Coverslipping
- Coverslip slides with an aqueous mounting medium, such as Fluoromount-G or ProlongGold. Take care to remove all bubbles using a cotton-tipped applicator.
Note: Other mounting agents could be used, however high bleed-through between dyes has been noted by some within days of coverslipping. - Use clear nail polish to seal the edges, preventing the sections from drying out due to evaporation. Allow nail polish to dry in a light-tight container while slides remain flat and at room temperature, and then store in a light-tight container wrapped in foil at 4 °C.
- Coverslip slides with an aqueous mounting medium, such as Fluoromount-G or ProlongGold. Take care to remove all bubbles using a cotton-tipped applicator.
3. Imaging the Stained Tissue
- Microscopy
- Allow the nail polish to dry for at least one hour before commencing microscopy, which should take place in a darkened room.
- Acquire photomicrographs using a confocal or research microscope with a fluorescent light source and a digital camera attachment. Using the accompanying software, set the exposure for each wavelength – 405, 488, and 594 – separately. Note: in-depth imaging instructions should be available online from the microscope manufacturer.
- Acquire photomicrographs in each channel without moving the sections or adjusting the focus. Take images in color, or alternately in grayscale and convert to color afterward.
Note: Color or grayscale images from each channel can be collated in post-processing. - Ensure that tissue sections are not exposed to ambient light or microscopic light for long periods of time, as photo-bleaching of the sections will occur. To avoid this, increase exposure time rather than increasing light/laser intensity.
- Do not turn off the fluorescent light source within 30 min of being turned on.
Note: Switching the source on and off in quick succession may decrease the life span of the fluorescent bulb.
Wyniki
This staining protocol results in rat brain tissue sections that have microglia fluorescently labeled in the 594 channel (red) and neurons labeled in the 488 channel (green; see Figure 4). If a nuclear stain has been done, it will show in the 405 channel (blue). Images can be taken in different channels and overlaid for direct comparison of the three channels, or between any two channels. Many digital acquisition software suites include this functionality. Double-labeling with Iba1 and Pan-neuronal marke...
Dyskusje
The overall goal of this communication was to introduce immunohistochemistry procedures to the reader. For this, the example of double-labeling with Iba1 and Pan-neuronal antigens to observe microglia and neurons in paraformaldehyde perfused, sucrose cryoprotected, cryosectioned rat brain was used.
This technique can be adapted to serve endless purposes. An array of different antigens in a variety of tissue types such as, but not limited to brain, lung, liver, kidney and intestine can be visua...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors would like to thank Mr. Ryan Hart and Mr. Arriz Lucas for their invaluable feedback on this communication. This work was supported by NIH NINDS R01 NS065052 and Phoenix Children’s Hospital Mission Support Funds.
Materiały
| Name | Company | Catalog Number | Comments |
| Fisherbrand Superfrost Plus Glass Slides | Fisher Scientific | 22-034-979 | Used for tissue mounting (1.2.2) |
| Oven | Thermo Scientific | 51028112 | Used for tissue drying (2.1.1) |
| Mini Pap pen | Life Technologies | 00-8877 | Used in step 2.2.2 |
| Andwin Scientific Tissue-tek Slide Staining Dish | Fisher Scientific | 22-149-429 | Used for all washes during staining (2.2), as well as the Hoechst step (2.2.8) |
| Kimwipes | Fisher Scientific | 06-666-A | Used for drying slides (2.2) |
| Black Staining Box | Ted Pella | 21050 | Used for blocking and staining steps (2.2) |
| Normal Donkey Serum | Fisher Scientific | 50-413-253 | Used for block and antibody incubation (2.2) |
| Mouse α-Pan-neuronal | Millipore | MAB2300 | Used for primary antibody (2.2.4) |
| Rabbit α-Iba1 | Wako Chemical | 019-19741 | Used for primary antibody (2.2.4) |
| Donkey α-rabbit 594 | Jackson ImmunoResearch | 711-585-152 | Used for secondary antibody (2.2.6) |
| Donkey α-mouse 488 | Jackson ImmunoResearch | 715-545-150 | Used for secondary antibody (2.2.6) |
| Caterer's foil | Any | N/A | Used in steps 1.2.2 and 2.3.2 |
| Fluoromount-G | Southern Biotech | 0100-01 | Used for coverslipping (2.2.8) |
| Coverslips | Fisher Scientific | 12544E | Used for coverslipping (2.2.8) |
| Clear Nail Polish | Any | N/A | Used for coverslipping (2.2.8) |
| Axio Observer.Z1 and LSM 710 (laser scanning, confocal) | Carl Zeiss | N/A | Used for imaging (3) |
| Axioskop A2 | Carl Zeiss | N/A | Used for imaging (3) |
| CitriSolv | FisherScientific | For DAB protocol | |
| ABC | Vector Laboratories | PK-6100 | For DAB protocol |
| DAB Peroxidase kit | Vector Laboratories | SK-4100 | For DAB protocol |
| Biotinylated horse α-rabbit IgG | Vector Laboratories | BA-1100 | For DAB protocol |
| Biotinylated horse α-mouse IgG | Vector Laboratories | BA-2001 | For DAB protocol |
| 30% Hydrogen Peroxide | FisherScientific | H325-500 | For DAB protocol |
| Wheaton slide racks and staining dishes | TedPella | 21043 | For DAB protocol |
| Masterflex perfusion pump and tubing | Cole-Parmer | Used for perfusion (1.1.1 and 1.1.2) | |
| Andwin scientific tissue-tek CRYO-OCT compound (case of 12) | Fisher Scientific | 14-373-65 | Used for tissue freezing (1.2.1) |
| Thermometer (-50 to 50 C) | Fisher Scientific | 15-059-228 | Used for tissue freezing (1.2.1) |
| Cryostat | Leica | CM3500S | Used for tissue sectioning (1.2.2) |
| Staining Dish, Plastic with 2 Lids | Grale Scientific | 353 | For antigen retrival |
| 20 Place Staining Rack, Slides Horizontal | Grale Scientific | 354 | For antigen retrival |
| Microwave | Any | N/A | For antigen retrival |
Odniesienia
- Marrack, J. R. Chemistry of antigens and antibodies. Nature. 134, 468-469 (1934).
- Coons, A. H., Creech, H. J., Jones, R. N., Berliner, E. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 45, 159-170 (1942).
- Marshall, J. M. Localization of adrenocorticotropic hormone by histochemical and immunochemical methods. The Journal of experimental medicine. 94, 21-30 (1951).
- Mellors, R. C. Histochemical demonstration of the in vivo localization of antibodies: antigenic components of the kidney and the pathogenesis of glomerulonephritis. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 3, 284-289 (1955).
- Nakane, P. K., Pierce, G. B. Enzyme-labeled antibodies: preparation and application for the localization of antigens. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 14, 929-931 (1966).
- Avrameas, S., Uriel, J. Method of antigen and antibody labelling with enzymes and its immunodiffusion application. Comptes rendus hebdomadaires des seances de l'Academie des sciences. Serie D: Sciences naturelles. 262, 2543-2545 (1966).
- Cuello, A. C. . Immunohistochemistry. , (1983).
- Junqueira, L. C. U., Mescher, A. L. . Junqueira's basic histology : text and atlas. , (2013).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. Journal of visualized experiments : JoVE. , (2012).
- Christensen, N. K., Winther, L., Kumar, G. L., Rudbeck, L. . Education Guide: Immunohistochemical (IHC) staining methods. , 103-108 (2009).
- Wang, G., Achim, C. L., Hamilton, R. L., Wiley, C. A., Soontornniyomkij, V. Tyramide signal amplification method in multiple-label immunofluorescence confocal microscopy). Methods. 18, 459-464 (1999).
- Feldengut, S., Del Tredici, K., Braak, H. Paraffin sections of 70-100 mum: a novel technique and its benefits for studying the nervous system. Journal of neuroscience methods. 215, 241-244 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone