Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Piglet Model of Neonatal Hypoxic-Ischemic Encephalopathy
W tym Artykule
Podsumowanie
Hypoxic-ischemic encephalopathy following perinatal asphyxia can be studied using animal models. We demonstrate the procedures necessary for establishing a piglet model of neonatal hypoxic-ischemic encephalopathy.
Streszczenie
Birth asphyxia, which causes hypoxic-ischemic encephalopathy (HIE), accounts for 0.66 million deaths worldwide each year, about a quarter of the world’s 2.9 million neonatal deaths. Animal models of HIE have contributed to the understanding of the pathophysiology in HIE, and have highlighted the dynamic process that occur in brain injury due to perinatal asphyxia. Thus, animal studies have suggested a time-window for post-insult treatment strategies. Hypothermia has been tested as a treatment for HIE in pdiglet models and subsequently proven effective in clinical trials. Variations of the model have been applied in the study of adjunctive neuroprotective methods and piglet studies of xenon and melatonin have led to clinical phase I and II trials1,2. The piglet HIE model is further used for neonatal resuscitation- and hemodynamic studies as well as in investigations of cerebral hypoxia on a cellular level. However, it is a technically challenging model and variations in the protocol may result in either too mild or too severe brain injury. In this article, we demonstrate the technical procedures necessary for establishing a stable piglet model of neonatal HIE. First, the newborn piglet (< 24 hr old, median weight 1500 g) is anesthetized, intubated, and monitored in a setup comparable to that found in a neonatal intensive care unit. Global hypoxia-ischemia is induced by lowering the inspiratory oxygen fraction to achieve global hypoxia, ischemia through hypotension and a flat trace amplitude integrated EEG (aEEG) indicative of cerebral hypoxia. Survival is promoted by adjusting oxygenation according to the aEEG response and blood pressure. Brain injury is quantified by histopathology and magnetic resonance imaging after 72 hr.
Wprowadzenie
Perinatal asphyxia is an acute and frequently unpredicted condition associated with hypoxic-ischemic encephalopathy (HIE). The overall goal of this protocol is to demonstrate a piglet survival model of perinatal hypoxic-ischemic encephalopathy. This model can be used to investigate the effect of various degrees of hypoxia-ischemia on the neonatal brain and of experimental treatments on neuropathology, magnetic resonance imaging and spectroscopy (MRI and MRS) and biomarkers in body fluids such as blood, cerebrospinal fluid and urine. The model has also proven useful for investigating the cardiovascular system, respiratory system, kidney and liver, all of which are affected in global hypoxia-ischemia.
Perinatal asphyxia is the result of compromised oxygen supply intrapartum or in the immediate post-partum period. Intrapartum hypoxic events account for 0.66 million deaths worldwide each year, about a quarter of the world’s 2.9 million neonatal deaths in 20123. In 2010 1.15 million babies were estimated to have developed neonatal encephalopathy following birth asphyxia4. HIE defined as encephalopathy in infants born after 34 weeks of gestation occurs in 1-3/1,000 live births5 in the industrialized world and up to 8.5/1,000 live births in developing countries4. The risk of death is 10-60%, and the risk of neurological handicap in survivors 30-100%6,7. 50.2 million disability adjusted life years (DALYs) are attributed to intrapartum hypoxic events4. Presently the only treatment other than supportive for HIE is post-hypoxic hypothermia. Thus, advancements in diagnostic procedures and treatment strategies are essential to improve the management of HIE8.
Improvements in the prognosis after perinatal asphyxia and management of neonatal brain injury are based on expanding knowledge of underlying disease mechanisms and possible treatments. Animal models of HIE are particularly useful as different clinical events may lead to HIE and the incidence in any single birth center is low5. An experimental setup in which the influence of biological variation can be minimized, is essential when testing new prognostic and diagnostic tools and treatment strategies. An animal model should approximate the clinical situation as closely as possible, thus contributing to the understanding of the pathological mechanisms underlying the induced injury and the dynamic process involved in the disease and it´s outcome9. Animal models of neonatal HIE have included a number of species, including rodents, lamb, and swine. In comparison, the newborn piglet has higher resemblance to a human newborn with respect to size, cardiovascular system10 and brain maturity at the time of delivery11,12. Monitoring, instrumentation and outcome evaluation in the piglet model is similar to that used in the clinical care of infants with HIE. Accordingly, there is a high degree of translation into newborn care from this model.
Piglets models of perinatal hypoxia and HIE are used by many groups and vary in a number of areas13. According to the purpose of the experiment, careful attention must be paid to the choice of medications, method of inducing hypoxia-ischemia, method of controlling insult duration and severity, post-insult resuscitation and care, and outcome evaluation. To avoid bias a randomized trial design should always be used in interventional studies.
The method applied when inducing hypoxic-ischemic injury is important. Global hypoxia leading to HIE often results in multi-organ failure involving brain, heart, lungs, kidney and liver. Depending on the outcomes evaluated, models of HIE should be based on global hypoxia and ischemia rather than rely on focal ischemia, e.g., by ligation of carotid arteries14. A recent paper applied a combination of hypoxia (FiO2 12%) and carotid artery compression while maintaining mean arterial blood pressure > 40 mm Hg2. Another group induced global hypoxia by 8% O2 until negative base excess > 20 mmol/L or mean arterial blood pressure (MABP) < 15 mm Hg, and sacrificed the animals at 4 hr15. Hypoxia has also been titrated by cardiac output (to 30-40% of baseline), MABP (to 30-35 mm Hg) and arterial pH (6.95-7.05)16.
Models of global hypoxia-ischemia titrated by aEEG suppression similar to the one presented in this report, have demonstrated encephalopathy that is clinically, electrophysiologically, and neuropathologically comparable to the condition found in the asphyxiated term infant17,18.
The degree of HIE induced is essential. A useful animal model of HIE must also allow for testing of new diagnostic procedures and treatment options. To enable this, the models should induce moderate HIE where there is a treatment potential as severe brain injury with little or no treatment potential would be less relevant when evaluating new treatments. Tolerance to hypoxia varies considerably between test animals. Previous studies have shown that a more consistent brain injury can be achieved and that more animals survive17,19 by individualizing the induced hypoxia according to each piglet’s cerebral response evaluated by amplitude integrated electroencephalography (aEEG) rather than using a set FiO2 value throughout the hypoxic event. The duration of aEEG suppression correlates to the degree of brain injury, with few histopathologic changes at < 20 min aEEG suppression and severe seizures increasing at > 45 min aEEG suppression. A recent review of neuroprotective treatments for HIE identified the need for survival models enabling behavioral outcome measures in animal models20.
There are numerous advantages of the presented HIE piglet model. It is based on a species where results are highly likely to translate to the human physiology. Global hypoxia-ischemia models multi-organ failure and titration of hypoxia-ischemia by aEEG induces a consistent degree of brain injury with survival clinical relevant outcomes such that biomarkers, MRI and behavior may be evaluated at relevant time points.
Piglet models of perinatal asphyxia and HIE have not only contributed significantly to current insight into HIE pathophysiology, but have also successfully preceded clinical trials, ultimately resulting in new treatments in humans. Piglet model studies played a key role in establishing hypothermia as a treatment for HIE21, and are used in neonatal resuscitation research22. Various groups have used piglet models when performing research within asphyxia and HIE, and studies include hypothermia23, alpha-melanocyte-stimulating hormone24, cardiac arrest25, tyrosine hydroxylase activity26, repeated hypoxic exposure27, NMDA receptor activity14, and near-infrared spectroscopy28.
The piglet HIE model presented in this report is technically challenging to work with, as minor adjustments during the course of the procedure may result in either too mild or too severe brain injury29,2. We found that the existing literature lacked sufficient detail to reproduce previously published models. Thus, we here demonstrate each step of the technical procedures necessary for the establishment of a piglet 72 hr survival model in this report, enabling researchers to establish this advanced model for the study of HIE.
Protokół
The present protocol was approved by the Danish Animal Experiments Inspectorate. All test animals were anesthetized throughout the procedures. Reproduction of this protocol must be carried out in accordance with national ethics and animal welfare guidelines, and approved by local ethics committees.
1. Animals
- Danish Landrace piglets < 24 hr old weighing approximately 1,500 - 2,000 g.
2. Anesthesia and Maintenance Fluids
- Prepare the equipment needed for anesthesia (Figure 1): A mask for sevoflurane administration, alcohol swabs, peripheral intravenous catheter, rubber band, syringes with saline, propofol (5 mg/kg), fentanyl (10 µg/kg), and procaine benzylpenicillin (15,000 IU/kg).
- Induce anesthesia by delivering 1-2% sevoflurane through a breathing mask.
- Assess the depth of anesthesia by assessing for palpebral and withdrawal reflexes. When certain that the piglet is deeply anesthetized, percutaneously insert a peripheral intravenous catheter into the ear vein.
- To confirm patency of the peripheral intravenous catheter, flush the catheter with 1-2 ml of sterile 0.9% saline. Administer bolus injections of propofol (5 mg/kg) and fentanyl (10 µg/kg). Subsequent to bolus injection administration flush the intravenous catheter a second time using 1-2 ml of sterile 0.9% saline.
- Place syringes with propofol (10 mg/ml) and fentanyl (10 µg/ml) in two separate syringe infusion pumps. Connect i.v. tubes from the two syringe pumps to a three-way stopcock joining infusions into a single line that is connected to the intravenous catheter. Start continuous i.v. infusion of propofol (4-12 mg/kg/hr) and fentanyl (10 μg/kg/hr). Once continuous infusions are running, do not give further bolus injections of propofol and fentanyl.
- Discontinue administration of the sevoflurane gas anesthesia.
- Inject procaine benzylpenicillin (15,000 IU/kg) subcutaneously or intramuscularly as per local guidelines for antibiotic prophylaxis. Repeat daily. The video shows s.c. administration, which in piglets has been shown to result in higher plasma concentration and longer half-life when compared to i.m. administration.
- Apply eye lubricant ointment to prevent drying of the eyes. Pull the lower eyelid down gently to form a pocket-like opening. Squeeze a small amount of ointment inside the pocket. Close eye to distribute ointment. Check for dryness hourly and reapply as needed.
- Initiate a continuous rate infusion of 5% dextrose/0.45% NaCl i.v. at 10 ml/kg/hr. Reduce rate to 5 ml/kg/hr during and after hypoxia. Adjust infusion rate to maintain blood glucose levels between 2-10 mmol/L.
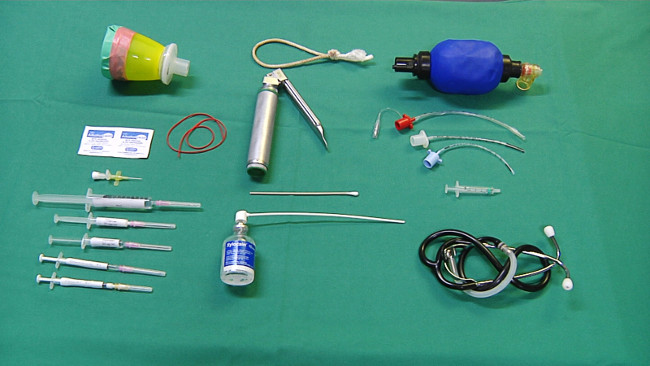
Figure 1. Equipment for anesthesia and intubation. Please click here to view a larger version of this figure.
3. Intubation and Ventilation
- Prepare the equipment needed for intubation (Figure 1): rocuronium bromide (1 mg/kg) for muscle relaxation, a rope sling (tie both ends of the same 4 mm nylon rope together to form a circle as depicted in Figure 1) for opening the mouth, a cotton tipped swab and veterinary laryngoscope with straight blade, xylocaine spray (100 mg/ml), various sized endotracheal tubes with cuff (sizes 3.0 mm, 2.5 mm, 2.0 mm), 500 ml self-inflating bag (bag valve mask) for ventilation, 2 ml syringe for cuff inflation, and stethoscope.
- Intubate by following the steps below:
- Place piglet in the supine position supporting the neck from each side to secure a straight laryngeal passage for intubation.
- Estimate the length of the endotracheal tube by measuring from the tip of the snout to the sternal notch (typically around 13 cm).
- Place rope sling around the upper jaw (facing down) and hold lower jaw and tongue upward to keep the mouth open.
- Administer rocuronium bromide (1 mg/kg) i.v. to induce muscle relaxation.
- Use laryngoscope to lift the tongue upwards.
- Use the cotton tipped swab to free the piglet’s long epiglottis, which may be either retroverted into the esophagus or caught behind the soft palate.
- Advance laryngoscope to keep the epiglottis lifted against the tongue base enabling full view of the arytenoid cartilages and vocal cords.
- Apply xylocaine spray (100 mg/ml) topically in the larynx to prevent laryngeal spasms.
- Advance endotracheal tube through the vocal cords. Use a rotating movement to aid the passing of the narrow tracheal cartilages. Advance tube according to the premeasured distance and connect to a self-inflating bag (bag valve mask) for manual ventilation. To help reduce friction during intubation spray the distal 1/3 of the endotracheal tube with xylocaine spray.
- To confirm correct endotracheal tube placement: observe for any indication of difficulty breathing, ausculate the chest listening for bilateral air entry into both lungs, visually confirm the presence of condensation in the proximal aspect of the endotracheal tube, and verify the presence of end tidal carbon dioxide with a colorimetric carbon dioxide detector or by expiratory CO2 reading on the mechanical ventilator if available. Normal end tidal carbon dioxide is around 5%. A value above 2% together with a normal-appearing waveform will confirm that the endotracheal tube is in the trachea.
- Inflate the endotracheal tube cuff to prevent aspiration. Cuff inflation pressure should be less than 25 cm H2O to avoid ischemic damage to the surrounding tissue. Holding the endotracheal tube in place, wind a piece of tape around the tube, close the jaw and continue taping around the snout to secure the tube in place. Gently pull on the tube to ensure that it stays in place.
- Connect endotracheal tube to mechanical ventilator.
- Adjust ventilator settings: volume-controlled ventilation, tidal volume (TV): 10 ml/kg [or peak inspiratory pressure (PIP) 15 cm with pressure controlled ventilation]. Positive end expiratory pressure (PEEP): 5 cm. I:E ratio 1:2. Respiratory rate: 35 (adjust rate to maintain end-tidal CO2 between 4.5-5.5 kPa).
4. Monitoring and Body Fluid Sampling
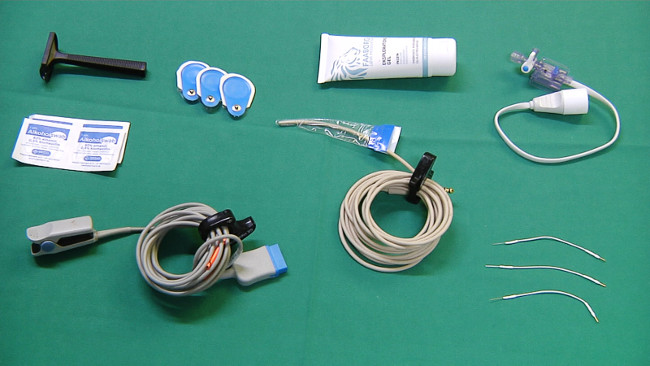
Figure 2. Equipment for monitoring. Please click here to view a larger version of this figure.
- Prepare the equipment needed for monitoring (Figure 2): adhesive tape, sterile lubricant, probe for continuous rectal temperature measurement, electrocardiogram (ECG) electrodes, pulse oximeter, razor, and EEG electrodes.
- Place saturation probe on a hind leg, lubricate rectal temperature probe and insert 6 cm into the rectum, place overhead radiant heater and/or heated inflatable air mattress to maintain the rectal temperature at the physiologic level of 38.5-39 °C, and place ECG electrodes.
- Place piglet in the prone position to shave areas of 1 cm x 1 cm for placement of subcutaneous needle EEG electrodes; one in front of each ear, and the reference electrode in the midline just behind the eyes. Clean the electrode site with an alcohol swab, then insert the needle electrode subcutaneously. Secure electrodes with adhesive tape and return piglet to the supine position.
- Turn on aEEG monitor.
NOTE: The amplitude integrated EEG consists of a dense trace with upper and lower margins. Lower and upper margins in this model are typically 15-50 μV, often higher than seen in infants. During hypoxia it is important to note, that artifact from the ECG may falsely elevate the aEEG trace. Bolus medications (propofol or fentanyl) may also transiently suppress the aEEG trace and should be avoided if possible during the experiment. Administration of drugs and clinical events should be marked to facilitate interpretation of the aEEG trace.
- Prepare the equipment needed for placing umbilical catheters for central arterial blood pressure monitoring and blood sampling (Figure 3): sterile gloves, sterile draping, scalpel, alcohol swabs, sterile wipes, umbilical vein catheter (5 Fr), umbilical artery catheter (3.5 Fr), suture set with forceps, curved micro forceps, scissors, needle holder and suture (e.g., size 3-0), transparent adhesive patch, 5 ml syringes for blood sampling.
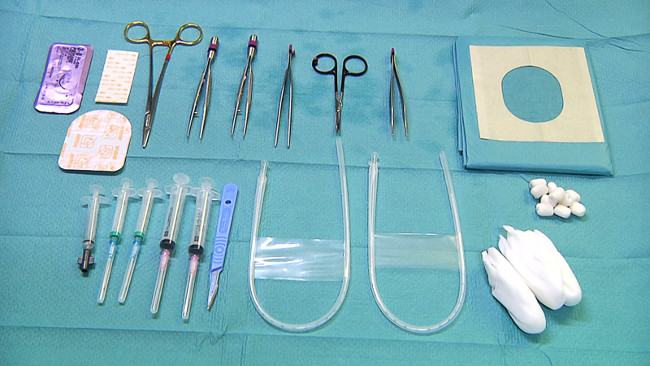
Figure 3. Equipment for umbilical lines and blood sampling. Please click here to view a larger version of this figure.
- Sterilize and drape the area around the umbilicus. Use a scalpel to cut the umbilical cord as close to the skin as possible. If this does not expose the umbilical vessels in the anaesthetized piglet, cut the umbilical skin 2 mm below the umbilical cord to expose the vessels. Identify the two small umbilical arteries and the one larger umbilical vein (Figure 4).
- Use curved micro forceps to dilate the artery (Figure 4) and insert the umbilical arterial catheter (3.5 Fr). Estimate insertion length in cm to 3 x weight (kg) + 10 (empirical formula based on correct placement in descending aorta above renal arteries on autopsy).
- Place a second 5 Fr catheter 5 cm into the umbilical vein (Figure 4). Verify intravascular catheter placement by blood return. Secure catheters by placing a purse-string suture around the umbilicus, pass the suture ends around each of the umbilical catheters and tie a knot. Cover catheters with transparent adhesive dressing.
- Collect blood samples at pre-specified time points: 1) immediately before hypoxia, 2) 30 min into hypoxic insult 3) at the end of the 45 min hypoxic insult 4) 2 hr after hypoxic insult. Depending on the purpose of the experiment other time points for blood samples may be chosen.
- Use arterial blood gas analysis from blood samples taken during hypoxia to verify blood gas changes caused by hypoxia-ischemia (Table 1). Note that piglets have low hemoglobin at birth (around 8 g/dL) and may become anemic from frequent blood sampling. Withdraw less than 2 ml blood/kg bodyweight per draw, less than 5 ml blood/kg bodyweight within 24 h and adhere to local guidelines for blood sampling. Signs of anemia include decreasing hematocrit and tachycardia.
- Connect arterial line to a monitor for continues intra-arterial blood pressure monitoring (MABP). Use venous line for fluid and drug administration.
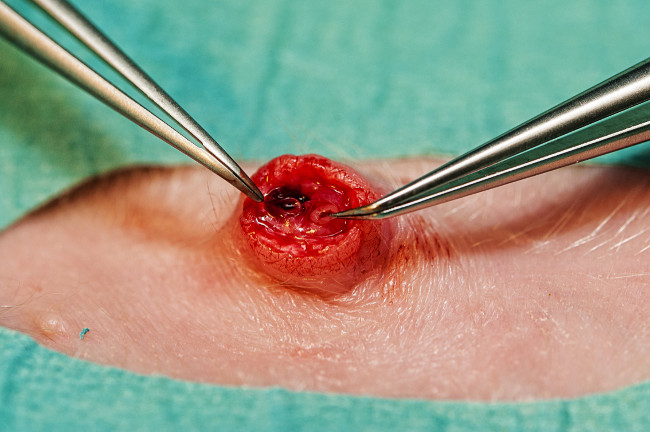
Figure 4. Umbilical vessels. Umbilical vein (right) and one of two umbilical arteries (left). Please click here to view a larger version of this figure.
5. Hypoxia
- Wait 60 min after completing monitoring. Induce hypoxia by switching to a 4% O2/96% N2 gas mixture. Monitor vital parameters and aEEG closely and continue hypoxia for 45 min.
- Once the aEEG trace is flat (upper margin <7 µV), adjust oxygenation by altering the inspired oxygen fraction (FiO2) and mean airway pressure to the highest FiO2 level maintaining a flat trace aEEG according to the flowchart shown in Figure 5. Aim for MABP below 70% of baseline for at least 10 min and if needed lower FiO2 to ensure hypotension and ischemia. The described level of hypotension has previously been shown by others19,13,30 to produce relevant hypoxic-ischemic brain injury.
- In case of severe hypotension (MABP <25 mm Hg) treat stepwise as follows: briefly increase FiO2 by 1-5%, saline bolus (10 ml/kg), infusion of dopamine (5-20 µg/kg/min), and infusion of noradrenaline (20 ng-1 µg/kg/min).
- In case of seizures lasting >10 min (clonic, tonic or myoclonic seizures, typically focal or as indicated by sudden changes in aEEG amplitude) treat stepwise with (allowing 30 min before proceeding to the next injection): slow bolus of phenobarbital i.v. 20 mg/kg, repeat phenobarbital i.v. 20 mg/kg, and midazolam i.v. 0.5 mg/kg.
- If seizures develop and are nonresponsive to drug therapy euthanasia is indicated.
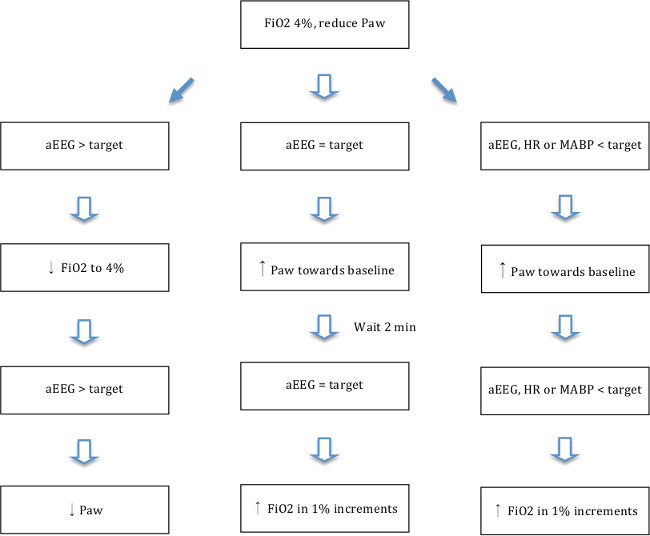
Figure 5. Hypoxia-ischemia flowchart. Flowchart showing adjustments in oxygenation (FiO2 and Paw) according to aEEG response. Mean airway pressure (Paw) was adjusted by changing PIP/TV (lower PIP gives lower Paw) and respiratory rate (lower RR gives lower Paw). Target aEEG = upper margin of trace < 5 µV and lower margin of trace > 3 µV (average 4 µV). Target heart rate (HR) = > 80. Target mean arterial blood pressure (MABP) = MABP > 25. Please click here to view a larger version of this figure.
6. 72 hr survival
- Monitor the piglet closely after the hypoxic insult, and gradually reduce the rates of infusion of propofol and fentanyl. Extubate at the point at which the animal is breathing voluntarily.
- During survival period, keep piglets at an animal facility with 24 hr watch by personnel trained in intensive care and monitoring. Administer i.v. glucose infusions 20 ml/kg 2-3 hourly. At 26-48 hr after hypoxia-ischemia partial bottle feeding may be initiated. Aspiration to the trachea and lungs may occur by bottle feeding if the gag reflex is not present.
- If relevant to the experiment, evaluate neurologic status using scoring system developed by Thoresen et al., described in detail in the original publication17.
7. Outcome Evaluation
- After 72 hr, anesthetize and ventilate again as described in section 2 and 3. Perform MRI scan with relevant imaging modalities as described by Munkeby et al31 and others2.
- At the end of the experiment euthanize piglets with a lethal dose of pentobarbital (5 g/kg i.v.).
- Prepare the brain for examination according to the goal of the experiment.
- For brain histology on formalin fixed tissue: use cardiac perfusion with 4% paraformaldehyde in PBS, followed by dissection and post-fixing in paraformaldehyde as described by Robertson et al.2 , Chakkarapani et al1 or Liu et al.18 or remove the brain and immerse in 4% paraformaldehyde as described by Andresen et al.32
- For analysis requiring snap-frozen tissue (e.g., RNA analysis or enzyme activity assays33): Remove the brain, dissect out brain regions of interest and snap-freeze tissue blocks of maximum 1 cm x 1 cm in liquid nitrogen as described by Munkeby et al.33
Wyniki
The effects of hypoxia-ischemia on the brain that occur during the induced insult are documented by recording the aEEG trace. A representative aEEG trace is shown in Figure 6.
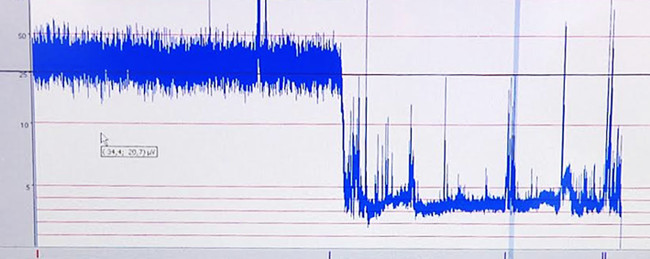
Figure 6. Representative aEEG trace. Low amplitude due to hypoxia-ischemia. Please click...
Dyskusje
Due to its complexity, the described model can only be implemented in facilities accredited and experienced in animal research. Approval by local ethics committees must be obtained prior to initiation of the experiments, and optimal animal welfare must be ensured at all times. As the model is based on the survival of the test animals, it is important that a sterile environment is maintained during invasive procedures to prevent infections.
The choice of anesthesia is important as most if not a...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors would like to thank John Kristensen and Søren Braad Andersen from the Department of Communication, Aarhus University Hospital, Denmark, for their exceptional help with filming and editing. Animal technician Diana Gyldenløve and veterinarian Birgitte Kousgaard, Institute of Clinical Medicine Aarhus University Hospital, Denmark for assisting with animal care. This study was supported by the Lundbeck Foundation, the Laerdal Foundation for Acute Medicine, Central Denmark Region’s Research Foundation, Augustinus Foundation, Aase and Ejnar Danielsens Foundation, the Institute of Clinical Medicine Aarhus University Hospital, Brødrene Hartmanns Foundation, Karen Elise Jensens Foundation, Fonden til Lægevidenskabens Fremme, and Marie Dorthea og Holger From, Haderslevs Fond.
Materiały
| Name | Company | Catalog Number | Comments |
| Warm-touch-pediatric blanket | Covidien | 5030840 | |
| Adhesive Apertrue Drape | Barrier | 915447 | |
| Utility Drape (sterile) 75x80 cm | Barrier | 800530 | |
| Neoflon | BD - Luer | 391350 | |
| Laryngoscope | Miller | 85-0045 | |
| Endotracheal tube 2.5 mm | Covidien | 111-25 | |
| Endotracheal tube 3.0 mm with cuff | Unomedical | MM61110030 | |
| Endotracheal tube 3.5 mm with cuff | Unomedical | MM61110035 | |
| Anesthesia machine | GE Healthcare | 1009-9002-000 | |
| EEG - electrodes/disposable subdermal needle electrode | Cephalon | ACCE120550 | |
| ECG - electrodes | medtronic | 3010107-003 | |
| ECG-electrodes for MR | philips | ACCE120550 | |
| Arterial blood sampler - aspirator | Radiometer medical ApS | 956552 | |
| Polyurethane Umbilical vein catheter (5 Fr/Ch) | Covidien | 8888160341 | |
| Polyurethane Umbilical vein catheter (3,5 Fr/ch) | Covidien | 8888160333 | |
| Suture set (size 3-0) | Covidien | 8886 623341 | |
| BD Spinal needle 0.7x38mm | BD needles | 405254 | |
| Gas with 96% Nitrogen / 4% oxygen | Air Liquide | made on order | |
| NeuroMonitor (CFM) system | Natus Medical Incorporated | OBM70002 |
Odniesienia
- Chakkarapani, E., et al. Xenon enhances hypothermic neuroprotection in asphyxiated newborn pigs. Annals of. 68, 330-341 (2010).
- Robertson, N. J., et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain : a journal of neurology. 136, 90-105 (2013).
- Lawn, J. E., et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet. 384, 189-205 (2014).
- Lee, A. C., et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatric research. 74, 50-72 (2013).
- Kurinczuk, J. J., White-Koning, M., Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early human development. 86, 329-338 (2010).
- Shankaran, S., Woldt, E., Koepke, T., Bedard, M. P., Nandyal, R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early human development. 25, 135-148 (1991).
- Robertson, C. M., Finer, N. N., Grace, M. G. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. The Journal of pediatrics. 114, 753-760 (1989).
- Bennet, L., Booth, L., Gunn, A. J. Potential biomarkers for hypoxic-ischemic encephalopathy. Seminars in feta., & neonatal medicine. 15, 253-260 (2010).
- Yager, J. Y., Ashwal, S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatric neurology. 40, 156-167 (2009).
- Buckley, N. M. Maturation of circulatory system in three mammalian models of human development. Comparative biochemistry and physiology. A, Comparative. 83, 1-7 (1986).
- Dobbing, J., Sands, J. Comparative aspects of the brain growth spurt. Early human development. 3, 79-83 (1979).
- Dobbing, J., Sands, J. Quantitative growth and development of human brain. Archives of disease in childhood. 48, 757-767 (1973).
- Foster, K. A., et al. An improved survival model of hypoxia/ischaemia in the piglet suitable for neuroprotection studies. Brain research. 919, 122-131 (2001).
- LeBlanc, M. H., Li, X. Q., Huang, M., Patel, D. M., Smith, E. E. AMPA antagonist LY293558 does not affect the severity of hypoxic-ischemic injury in newborn pigs. Stroke; a journal of cerebral circulation. 26, 1908-1914 (1995).
- Andresen, J. H., et al. Nicotine affects the expression of brain-derived neurotrophic factor mRNA and protein in the hippocampus of hypoxic newborn piglets. J Perinat Med. 37, 553-560 (2009).
- Cheung, P. Y., Gill, R. S., Bigam, D. L. A swine model of neonatal asphyxia. Journal of visualized experiments : JoVE. , (2011).
- Thoresen, M., et al. A piglet survival model of posthypoxic encephalopathy. Pediatric research. 40, 738-748 (1996).
- Liu, X., Tooley, J., Loberg, E. M., Suleiman, M. S., Thoresen, M. Immediate hypothermia reduces cardiac troponin I after hypoxic-ischemic encephalopathy in newborn pigs. Pediatric research. 70, 352-356 (2011).
- Bjorkman, S. T., et al. Hypoxic/Ischemic models in newborn piglet: comparison of constant FiO2 versus variable FiO2 delivery. Brain research. 1100, 110-117 (2006).
- Robertson, N. J., et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant. The Journal of pediatrics. 160, 544-552 (2012).
- Jacobs, S. E., et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 1, CD003311 (2013).
- Andresen, J. H., et al. Resuscitation with 21 or 100% oxygen in hypoxic nicotine-pretreated newborn piglets: possible neuroprotective effects of nicotine. Neonatology. 93, 36-44 (2008).
- Karlsson, M., et al. Delayed hypothermia as selective head cooling or whole body cooling does not protect brain or body in newborn pig subjected to hypoxia-ischemia. Pediatric research. 64, 74-78 (2008).
- Kovacs, J., et al. Asphyxia-induced release of alpha-melanocyte-stimulating hormone in newborn pigs. Peptides. 22, 1049-1053 (2001).
- Varvarousi, G., et al. Asphyxial cardiac arrest, resuscitation and neurological outcome in a Landrace/Large-White swine model. Laboratory animals. 45, 184-190 (2011).
- Tammela, O., Pastuszko, A., Lajevardi, N. S., Delivoria-Papadopoulos, M., Wilson, D. F. Activity of tyrosine hydroxylase in the striatum of newborn piglets in response to hypocapnic hypoxia. Journal of neurochemistry. 60, 1399-1406 (1993).
- Cote, A., Barter, J., Meehan, B. Age-dependent metabolic effects of repeated hypoxemia in piglets. Canadian journal of physiology and pharmacology. 78, 321-328 (2000).
- Tichauer, K. M., et al. Assessing the severity of perinatal hypoxia-ischemia in piglets using near-infrared spectroscopy to measure the cerebral metabolic rate of oxygen. Pediatric research. 65, 301-306 (2009).
- Jasani, M. S., Salzman, S. K., Tice, L. L., Ginn, A., Nadkarni, V. M. Anesthetic regimen effects on a pediatric porcine model of asphyxial arrest. Resuscitation. 35, 69-75 (1997).
- Chakkarapani, E., Thoresen, M., Liu, X., Walloe, L., Dingley, J. Xenon offers stable haemodynamics independent of induced hypothermia after hypoxia-ischaemia in newborn pigs. Intensive care medicine. 38, 316-323 (2012).
- Munkeby, B. H., et al. A piglet model for detection of hypoxic-ischemic brain injury with magnetic resonance imaging. Acta radiologica. 49, 1049-1057 (2008).
- Andresen, J. H., et al. Newborn piglets exposed to hypoxia after nicotine or saline pretreatment: long-term effects on brain and heart. J Matern Fetal Neonatal Med. 22, 161-168 (2009).
- Munkeby, B. H., et al. Resuscitation with 100% O2 increases cerebral injury in hypoxemic piglets. Pediatric research. 56, 783-790 (2004).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone