Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Preparation of a Corannulene-functionalized Hexahelicene by Copper(I)-catalyzed Alkyne-azide Cycloaddition of Nonplanar Polyaromatic Units
W tym Artykule
Podsumowanie
Here, we present a protocol to synthesize a complex organic compound comprised of three nonplanar polyaromatic units, assembled easily with reasonable yields.
Streszczenie
The main purpose of this video is to show 6 reaction steps of a convergent synthesis and prepare a complex molecule containing up to three nonplanar polyaromatic units, which are two corannulene moieties and a racemic hexahelicene linking them. The compound described in this work is a good host for fullerenes. Several common organic reactions, such as free-radical reactions, C-C coupling or click chemistry, are employed demonstrating the versatility of functionalization that this compound can accept. All of these reactions work for planar aromatic molecules. With subtle modifications, it is possible to achieve similar results for nonplanar polyaromatic compounds.
Wprowadzenie
Due to their special geometry, corannulene and helicenes are molecules that can adopt a structure far from planarity and give rise to interesting properties.1-15 In the last few years, the search of molecular receptors for carbon nanotubes and fullerenes is a very active area16-19 due, mainly, to their potential applications as materials for organic solar cells, transistors, sensors and other devices.20-28 The excellent complementarity in shape between corannulene and a fullerene have attracted the attention of several researchers with the aim of designing molecular receptors capable of establishing supramolecular association by dispersion forces.29-39
The chemistry of the above mentioned nonplanar polyaromatic compounds is similar to that described for totally planar molecules, but it is sometimes difficult to find suitable conditions to achieve desired selectivities and yields.40 In this work we present the synthesis of a molecule (7) having three polyaromatic units in a few steps with good yields by applying easy and typical techniques found in every research laboratory. The molecule is of great importance because it can adopt a pincer-like conformation to establish good interactions with C6037 in solution; and it may open a research line as a potential receptor for higher chiral fullerenes thanks to the helicene linker, which is a chiral molecule due to the existence of a stereogenic axis.41-45 However, only racemic helicene will be used in this work.
At this point, the only limitation to synthesize these receptors is the preparation of helicenes and corannulenes, since they are not commercially available. But, according to new methods published elsewhere46-48 they can be obtained in suitable amounts in a reasonable short period of time.
Protokół
1. Functionalization of 2,15-Dimethylhexahelicene
- Dibromination of 2,15-dimethylhexahelicene
- Weigh 0.356 g (1.0 mmol) of 2,15-dimethylhexahelicene, 0.374 g (2.1 mmol) of freshly recrystallized N-bromosuccinimide (NBS) and 24 mg (0.07 mmol) of benzoyl peroxide (BPO) (70% wt with 30% of water as stabilizer). Place all solids in a 100 ml Schlenk flask with a magnetic stir bar. Put under nitrogen atmosphere by three cycles of gas evacuation followed by refilling with inert gas in the Schlenk line.
- Add 21 ml of carbon tetrachloride (CCl4). Degas the solution by the same evacuation/refilling process (step 1.1.1) with vigorous stirring and carefully in order to prevent massive loss of solvent.
- Heat at reflux (77 °C) the mixture with an oil bath for 4 hours. Check the reaction by 1H-nuclear magnetic resonance (NMR). Doublets between 3.7 ppm and 4.0 ppm should appear. They indicate the presence of diastereotopic -CH2- groups (Figure 1).
- Once finished, cool the mixture to room temperature and remove the solvent under vacuum. Set up a trap filled with liquid nitrogen to avoid pump contamination.
- Redissolve the crude in 30 ml of dichloromethane (DCM), transfer to a round-bottom flask and mix with 4 g of silica gel (typically add 5-fold the crude weight). Concentrate the mixture in a rotary evaporator.
- In the meantime, fill a column (length around 20 cm and a thickness of 4.5 cm) with SiO2 gel mixed previously with hexane/ethyl acetate (95:5) as the mobile phase. Add the mixture to the top of the column and then add a layer of sand (2 cm).
- Carefully pour in the new mobile phase and perform the chromatography by collecting fractions in test tubes (typically 20 ml per tube and 4 ml near the expected product elution). Check fractions by thin layer chromatography (TLC) with the same mobile phase (hexane/ethyl acetate 95:5) and image under UV light. The expected product (4b) should elute at a Retention factor (Rf) of 0.35 as a yellow oil after combining all wanted fractions and removing off the solvent in the rotary evaporator. 334 mg should be obtained (yield 65%).
NOTE: All Schlenk techniques, the use of an oil bath for heating and column chromatography settings will be widely utilized in most of the protocols, so from now on, they will not be covered in detail and only a few comments, when necessary, will be given.
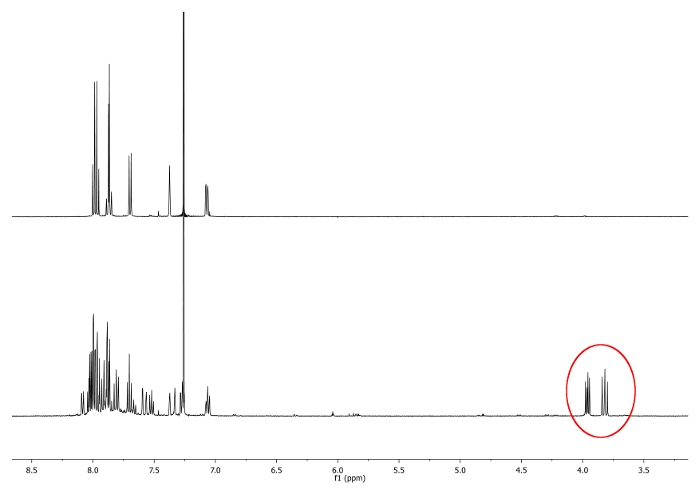
Figure 1. 1H-NMR spectra (500 MHz, CDCl3) of 2,15-dimethylhelicene (top) and an aliquot taken after 2 hr. New signals, corresponding to -CH2-, are depicted in a red circle (bottom). Please click here to view a larger version of this figure.
- Synthesis of 2,15-bis(azidomethyl)hexahelicene
- Weigh 0.103 g (0.2 mmol) of 2,15-bis(bromomethyl)hexahelicene and 0.390 g (6 mmol) of sodium azide. Place both solids in a 50 ml Schlenk flask equipped with magnetic bar and put under nitrogen atmosphere.
- Mix 8.6 ml of tetrahydrofuran (THF) with 5.2 ml of water (H2O) and pour the mixture of solvents into the Schlenk flask. Degas the solution.
- Heat at reflux (65 °C) for 3 hr. Check the reaction by 1H-NMR. -CH2- signals should shift to 3.75 ppm (Figure 2).
- Afterwards, cool down the mixture to room temperature and remove THF under vacuum. Dilute with 50 ml of H2O.
- Transfer the mixture to a separatory funnel and extract three times with 40 ml of DCM. Combine all organic phases and wash with pure H2O (50 ml).
- Purify the crude by column chromatography on silica gel using hexane/ethyl acetate (85:15) as the mobile phase to give a yellow oil at Rf=0.38 corresponding to 2,15-bis(azomethyl)hexahelicene (5b). 70 mg should be obtained (yield 80%).
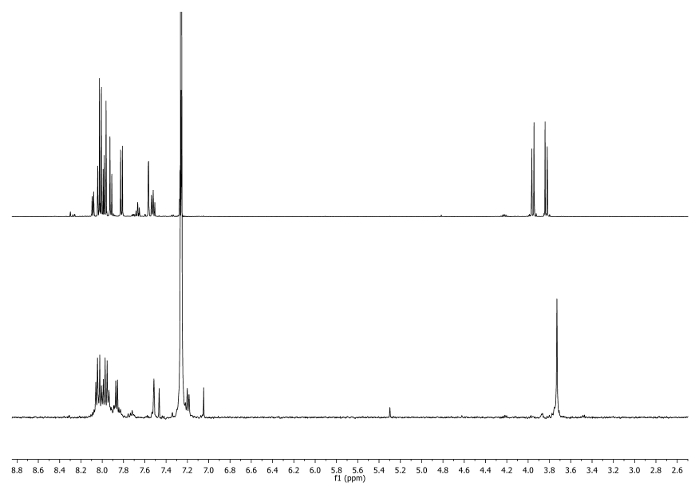
Figure 2: 1H-NMR spectra (500 MHz, CDCl3) of 4b (top) and an aliquot taken after 3 hr (bottom). Note the changes in the aliphatic region. Please click here to view a larger version of this figure.
2. Functionalization of Corannulene
- Monobromination of Corannulene
- Weigh 0.125 g (0.5 mmol) of corannulene, 89 mg (0.5 mmol) of freshly recrystallized NBS and 17 mg of gold(III) chloride hydrate.
- Place all compounds into a 10 ml vial specially designed for microwave reactions equipped with a magnetic bar and then put into a nitrogen atmosphere with the help of a 2-necked round-bottom flask.
- Add 7 ml of 1,2-dichloroethane (DCE) and degas the solution.
- Sonicate the mixture for 2 min to disperse gold salt particles.
- Heat inside the microwave reactor at 100 °C for 2 hr.
- When finished, transfer the crude to a round-bottom flask and remove solvent by rotary evaporation.
- Purify the crude by column chromatography on SiO2 gel using hexane as mobile phase.
NOTE: Bromocorannulene (4a) is obtained as a yellow solid at Rf=0.38. 99 mg should be obtained (yield 60%). Unreacted corannulene (3a) can be recovered and stored for further uses. It appears at Rf=0.29.
- Sonogashira Coupling of Bomocorannulene and Ethynyltrimethylsilane
- Weigh 49 mg (0.15 mmol) of bromocorannulene, 11 mg (0.015 mmol) of [PdCl2(dppf)]49,50 (dppf being 1,1-'bis(diphenylphsphino)ferrocene, 3 mg (0.015 mmol) of CuI.51
- Place all solids in a 50 ml Schlenk flask along with a magnetic bar and put under nitrogen atmosphere.
- Add 5.0 ml of triethylamine (NEt3) and degas the mixture.
- Finally, add 104 µl (0.75 mmol) of ethynyltrimethylsilane.
- Sonicate the mixture for 2 min to disperse metal salt particles.
- Heat at 85 °C for 24 hr with periodic sonication to prevent deposition of metal salts.
NOTE: The mixture color turned to black soon, indicating the presence of palladium(0). - Cool down to room temperature and evaporate NEt3 in vacuo.
- Redissolve in 20 ml of DCM and purify by column chromatography on silica gel eluting with hexane to give a yellow solid at Rf=0.28 corresponding to 5a. 41 mg should be obtained (yield 78%).
NOTE: If the crude is filtered through a Celite pad in DCM, a reasonable pure sample might be obtained, however phosphine derivatives are not removed totally.
- Preparation of Ethynylcorannulene by TMS Deprotection
- Weigh 35 mg (0.10 mmol) of 5a and 7.3 mg (0.125 mmol) of anhydrous potassium fluoride.
- Place all solids in a 50 ml Schlenk flask equipped with a magnetic bar and put under nitrogen atmosphere.
- Mix 4 ml of THF and 4 ml of methanol (MeOH) and pour the mixture into the Schlenk flask. Degas thoroughly.
- Allow to react at room temperature, Keep the flask away from light by covering it with an opaque film. Check the reaction by 1H-NMR by looking at 3.48 ppm. A signal corresponding to -CCH must emerge (Figure 3).
NOTE: Although this compound bears a terminal alkyne that is reactive and decomposes easily, we found no problems during the work up described below. It was carried out under natural light. - Once finished, remove THF under vacuum and dilute with 10 ml of water, transferring everything to a separatory funnel.
- Extract with DCM (3 x 15 ml), combine all organic phases in a round-bottom flask and concentrate in a rotary evaporator at room temperature to finally get a yellow solid corresponding to 6a. 27 mg should be obtained (quantitative yield).
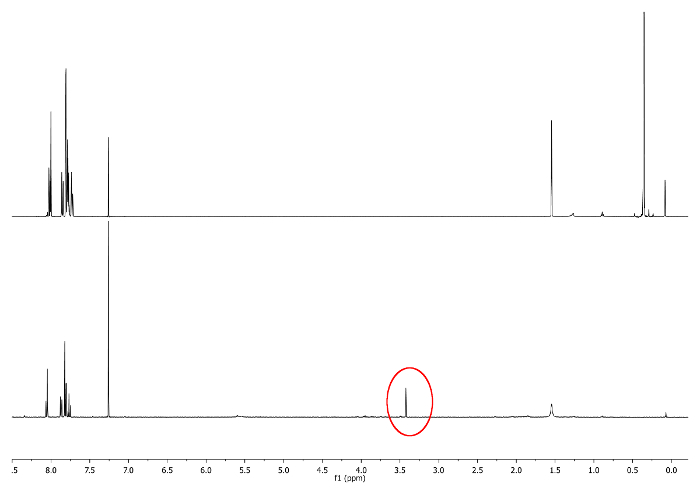
Figure 3: 1H-NMR spectra (500 MHz, CDCl3) of 5a (top) and 6a (bottom). -CCH singlet is depicted in a red circle. Please click here to view a larger version of this figure.
3. Final Assembly by Click Chemistry
- Weigh 15.3 mg (0.035 mmol) of 5b, 20.0 mg (0.073 mmol) of 6a, 1.4 mg (0.007 mmol) of ascorbic acid sodium salt, 1.7 mg (0.007 mmol) of CuSO4·5H2O.
- Place all solids in a 50 ml Schlenk flask equipped with a magnetic bar and put under nitrogen atmosphere.
- Mix 3 ml of H2O and 12 ml of THF and pour the mixture into the Schlenk flask. Degas the solution thoroughly.
- Heat at 65 °C for 3 days with a condenser connected to the top of the flask and check periodically the reaction to control temperature, stirring and solvent volume. Check the reaction by 1H-NMR. The signal at 3.48 ppm should disappear and be shifted to 7.27 ppm indicating the consumption of ethynyl corannulene and the existence of the triazole unit (Figure 4).
- When finished, remove THF under vacuum and dilute with 20 ml of water, transferring the mixture to a separatory funnel.
- Extract with DCM (3 x 20 ml), combine all organic phases in a round-bottom flask and concentrate in a rotary evaporator.
- Purify the crude by column chromatography on SiO2 gel eluting with hexane/ethyl acetate (1:1) to give a pale yellow solid at Rf = 0.59 corresponding to 7. 27 mg should be obtained (yield 75%).
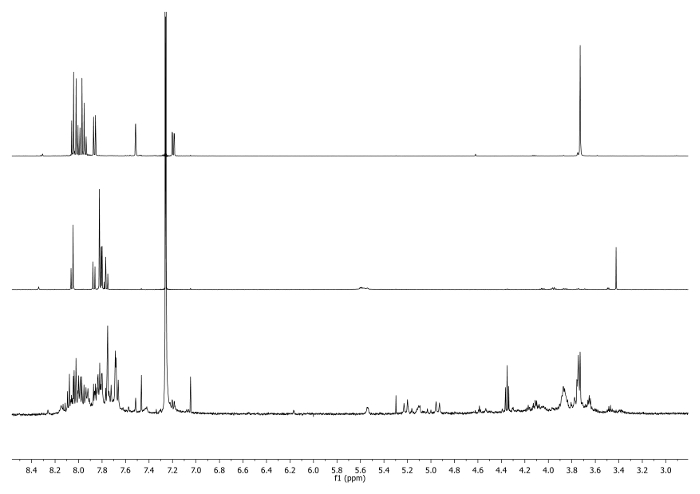
Figure 4: 1H-NMR spectra (500 MHz, CDCl3) of 5b (top), 6a (middle) and an aliquot taken after 2 days (bottom). Note the disappearance of -CCH signal in the crude. Please click here to view a larger version of this figure.
Wyniki
Corannulene (3a) and 2,15-dimethylhexahelicene (3b) could be prepared following current methods46-48 in a straightforward fashion with very good yields (Figure 5). Both share a common molecule, 2,7-dimethylnaphthalene, as the starting material, giving rise to a divergent to convergent synthesis of the final molecule.
Dyskusje
Final compound 7 has been prepared after 6 steps from nonplanar polyaromatic precursors 3a and 3b with moderate to very good yields at each reaction. The main limitation observed in this route was the bromination of both nonplanar polyaromatic compounds. However, in the case of compound 4a, an important amount of free corannulene can be recovered for further uses. The synthesis of 4
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was funded by the Spanish Ministerio de Economìa y Competitividad (CTQ 2013-41067-P). H.B. acknowledge with thanks a MEC-FPI grant.
Materiały
| Name | Company | Catalog Number | Comments |
| 2,15-Dimethylhexahelicene | N/A | N/A | Prepared according to reference 5b,c in the main text. |
| Corannulene | N/A | N/A | Prepared according to reference 5a in the main text. |
| N-Bromosuccinimide (NBS) | Sigma Aldrich | B8.125-5 | ReagentPlus®, 99%. Recrystallized from hot water. |
| Benzoyl peroxide (BPO) | Sigma Aldrich | B-2030 | ~70% (titration). 30% water as stabilizer. |
| Sodium azide | Sigma Aldrich | S2002 | ReagentPlus®, ≥99.5%. |
| Gold (III) chloride Hydrate | Sigma Aldrich | 50778 | puriss. p.a., ACS reagent, ≥49% Au basis. |
| Ethynyltrimethylsilane | Sigma Aldrich | 218170 | 98%. |
| [PdCl2(dppf)] | N/A | N/A | Prepared according to reference 6 in the main text. |
| CuI | N/A | N/A | Prepared according to reference 7 in the main text. |
| KF | Sigma Aldrich | 307599 | 99%, spray-dried. |
| (+)-Sodium L-ascorbate | Fluka | 11140 | BioXtra, ≥99.0% (NT). |
| Copper(II) Sulphate 5-hydrate | Panreac | 131270 | for analysis. |
| Carbon tetrachloride (CCl4) | Fluka | 87030 | for IR spectroscopy, ≥99.9%. |
| Dichloromethane (DCM) | Fisher Scientific | D/1852/25 | Analytical reagent grade. Distilled prior to use. |
| Hexane | Fisher Scientific | H/0355/25 | Analytical reagent grade. Distilled prior to use. |
| Ethyl acetate | Scharlau | AC0145025S | Reagent grade. Distilled prior to use. |
| Tetrahydrofuran (THF) | Fisher Scientific | T/0701/25 | Analytical reagent grade. Distilled prior to use. |
| 1,2-Dichloroethane (DCE) | Sigma Aldrich | D6,156-3 | ReagentPlus®, 99%. |
| Methanol (MeOH) | VWR | 20847.36 | AnalaR NORMAPUR. |
| Triethyl amine (NEt3) | Sigma Aldrich | T0886 | ≥99%. |
| Silica gel | Acros | 360050010 | Particle size 40-60mm. |
| Sand - low iron | Fisher Scientific | S/0360/63 | General purpose grade. |
| TLC Silica gel 60 F254 | Merck | 1.05554.0001 | |
| Monowave 300 (Microwave reactor) | Anton Para | ||
| Sonicator | Grupo Selecta | 3000513 | 6 Litres. |
Odniesienia
- Scott, L. T., Hashemi, M. M., Bratcher, M. S. Corannulene bowl-to-bowl inversion is rapid at room temperature. J. Am. Chem. Soc. 114 (5), 1920-1921 (1992).
- Sygula, A., et al. Bowl stacking in curved polynuclear aromatic hydrocarbons: crystal and molecular structure of cyclopentacorannulene. J. Chem. Soc., Chem. Commun. (22), 2571-2572 (1994).
- Nuckolls, C., et al. Circular Dichroism and UV−Visible Absorption Spectra of the Langmuir−Blodgett Films of an Aggregating Helicene. J. Am. Chem. Soc. 120 (34), 8656-8660 (1998).
- Beljonne, D., et al. Electro-optic response of chiral helicenes in isotropic media. J. Chem. Phys. 108 (4), 1301-1304 (1998).
- Treboux, G., Lapstun, P., Wu, Z., Silverbrook, K. Electronic conductance of helicenes. Chem. Phys. Lett. 301 (5-6), 493-497 (1999).
- Katz, T. J. Syntheses of Functionalized and Aggregating Helical Conjugated Molecules. Angew. Chem., Int. Ed. 39 (11), 1921-1923 (2000).
- Furche, F., et al. Circular Dichroism of Helicenes Investigated by Time-Dependent Density Functional Theory. J. Am. Chem. Soc. 122 (8), 1717-1724 (2000).
- Urbano, A. Recent Developments in the Synthesis of Helicene-Like Molecules. Angew. Chem., Int. Ed. 42 (34), 3986-3989 (2003).
- Botek, E., Champane, B., Turki, M., André, J. M. Theoretical study of the second-order nonlinear optical properties of [N]helicenes and [N]phenylenes. J. Chem. Phys. 120 (4), 2042-2048 (2004).
- Lovas, F. J., et al. Interstellar Chemistry: A Strategy for Detecting Polycyclic Aromatic Hydrocarbons in Space. J. Am. Chem. Soc. 127 (12), 4345-4349 (2005).
- Wigglesworth, T. J., Sud, D., Norsten, T. B., Lekhi, V. S., Branda, N. R. Chiral Discrimination in Photochromic Helicenes. J. Am. Chem. Soc. 127 (20), 7272-7273 (2005).
- Wu, Y. -. T., Siegel, J. S. Aromatic Molecular-Bowl Hydrocarbons: Synthetic Derivatives, Their Structures, and Physical Properties. Chem. Rev. 106 (12), 4843-4867 (2006).
- Tsefrikas, V. M., Scott, L. T. Geodesic Polyarenes by Flash Vacuum Pyrolysis. Chem. Rev. 106 (12), 4868-4884 (2006).
- Wu, Y. -. T., Hayama, T., Baldrige, K. K., Linden, A., Siegel, J. S. Synthesis of Fluoranthenes and Indenocorannulenes: Elucidation of Chiral Stereoisomers on the Basis of Static Molecular Bowls. J. Am. Chem. Soc. 128 (21), 6870-6884 (2006).
- Wu, Y. -. T., Siegel, J. S. Synthesis, structures, and physical properties of aromatic molecular-bowl hydrocarbons. Top. Curr. Chem. 349, 63-120 (2014).
- Pérez, E. M., Martìn, N. Curves ahead: molecular receptors for fullerenes based on concave-convex complementarity. Chem. Soc. Rev. 37 (8), 1512-1519 (2008).
- Tashiro, K., Aida, T. Metalloporphyrin hosts for supramolecular chemistry of fullerenes. Chem. Soc. Rev. 36 (2), 189-197 (2007).
- Kawase, T. Ball- Bowl- and Belt-Shaped Conjugated Systems and Their Complexing Abilities: Exploration of the Concave−Convex π−π Interaction. Chem. Rev. 106 (12), 5250-5273 (2006).
- Martin, N., Pérez, E. M. Molecular tweezers for fullerenes. Pure Appl. Chem. 82 (3), 523-533 (2010).
- Hoppe, H., Sariciftci, N. S. Morphology of polymer/fullerene bulk heterojunction solar cells. J. Mater. Chem. 16 (1), 45-61 (2006).
- Kim, S. N., Rusling, J. F., Papadimitrakopoulos, F. Carbon Nanotubes for Electronic and Electrochemical Detection of Biomolecules. Adv. Mater. 19 (20), 3214-3228 (2007).
- Dennler, G., Scharber, M. C., Brabec, C. J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 21 (13), 1323-1338 (2009).
- Helgesen, M., Søndergaard, R., Krebs, F. C. Advanced materials and processes for polymer solar cell devices. J. Mater. Chem. 20 (1), 36-60 (2010).
- Brabec, C. J., et al. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 22 (34), 3839-3856 (2010).
- Delgado, J. L., Bouit, P. -. A., Filippone, S., Herranz, M. A., Martìn, N. Organic photovoltaics: a chemical approach. Chem. Commun. 46 (27), 4853-4865 (2010).
- Schnorr, J. M., Swager, T. M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 23 (3), 646-657 (2011).
- Wang, C., Takei, K., Takahashi, T., Javey, A. Carbon nanotube electronics - moving forward. Chem. Soc. Rev. 42 (7), 2592-2609 (2013).
- Park, S., Vosguerichian, M., Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale. 5, 1727-1752 (2013).
- Mizyed, S., et al. Embracing C60 with Multiarmed Geodesic Partners. J. Am. Chem. Soc. 123 (51), 12770-12774 (2001).
- Sygula, A., Sygula, R., Ellern, A., Rabideau, P. W. Novel Twin Corannulene: Synthesis and Crystal Structure Determination of a Dicorannulenobarrelene Dicarboxylate. Org. Lett. 5 (15), 2595-2597 (2003).
- Georghiou, P. E., Tran, A. H., Mizyed, S., Bancu, M., Scott, L. T. Concave Polyarenes with Sulfide-Linked Flaps and Tentacles: New Electron-Rich Hosts for Fullerenes. J. Org. Chem. 70 (16), 6158-6163 (2005).
- Sygula, A., Fronczek, F. R., Sygula, R., Rabideau, P. W., Olmstead, M. M. A Double Concave Hydrocarbon Buckycatcher. J. Am. Chem. Soc. 129 (13), 3842-3843 (2007).
- Yanney, M., Sygula, A. Tridental molecular clip with corannulene pincers: is three better than two?. Tetrahedron Lett. 54 (21), 2604-2607 (2013).
- Stuparu, M. C. Rationally Designed Polymer Hosts of Fullerene. Angew. Chem., Int. Ed. 52 (30), 7786-7790 (2013).
- Le, V. H., Yanney, M., McGuire, M., Sygula, A., Lewis, E. A. Thermodynamics of Host-Guest Interactions between Fullerenes and a Buckycatcher. J. Phys. Chem. B. 118 (41), 11956-11964 (2014).
- Álvarez, C. M. Enhanced association for C70 over C60 with a metal complex with corannulene derivate ligands. Dalton Trans. 43 (42), 15693-15696 (2014).
- Álvarez, C. M. Assembling Nonplanar Polyaromatic Units by Click Chemistry. Study of Multicorannulene Systems as Host for Fullerenes. Org. Lett. 17 (11), 2578-2581 (2015).
- Yanney, M., Fronczek, F. R., Sygula, A. A 2:1 Receptor/C60 Complex as a Nanosized Universal Joint. Angew. Chem. Int. Ed. 54 (38), 11153-11156 (2015).
- Kuragama, P. L. A., Fronczek, F. R., Sygula, A. Bis-corannulene Receptors for Fullerenes Based on Klärner's Tethers: Reaching the Affinity Limits. Org. Lett. 17 (21), (2015).
- George, S. R. D., Frith, T. D. H., Thomas, D. S., Harper, J. B. Putting corannulene in its place. Reactivity studies comparing corannulene with other aromatic hydrocarbons. Org. Biomol. Chem. 13 (34), 9035-9041 (2015).
- Shen, Y., Chen, C. -. F. Helicenes: Synthesis and Applications. Chem. Rev. 112 (3), 1463-1535 (2012).
- Crassous, J., Saleh, N., Shen, C. Helicene-based transition metal complexes: synthesis, properties and applications. Chem. Sci. 5 (10), 3680-3694 (2014).
- Nakamura, K., Furumi, S., Takeuchi, M., Shibuya, T., Tanaka, K. Enantioselective Synthesis and Enhanced Circularly Polarized Luminescence of S-Shaped Double Azahelicenes. J. Am. Chem. Soc. 136 (15), 5555-5558 (2014).
- Schweinfurth, D., Zalibera, M., Kathan, M., Shen, C., Mazzolini, M., Trapp, N., Crassous, J., Gescheidt, G., Diederich, F. Helicene Quinones: Redox-Triggered Chiroptical Switching and Chiral Recognition of the Semiquinone Radical Anion Lithium Salt by Electron Nuclear Double Resonance Spectroscopy. J. Am. Chem. Soc. 136 (37), 13045-13052 (2014).
- Šámal, M., Chercheja, S., Rybáček, J., Vacek Chocholoušová, J., Vacek, J., Bednárová, L., Šaman, D., Stará, I. G., Starý, I. An Ultimate Stereocontrol in Asymmetric Synthesis of Optically Pure Fully Aromatic Helicenes. J. Am. Chem. Soc. 137 (26), 8469-8474 (2015).
- Siegel, J. S., Butterfield, A. M., Gilomen, B. Kilogram scale production of corannulene. Organic Process Research & Development. 16 (4), 664-676 (2012).
- Mallory, F. B., Mallory, C. W. Photocyclization of stilbenes and related molecules. Organic Reactions. , (1984).
- Sato, M., et al. Convenient synthesis and reduction properties of [7] circulene. J. Chem. Soc., Perkin Trans. 2. (9), 1909-1914 (1998).
- Anderson, G. K., Lin, M. Bis(Benzonitrile)dichloro complexes of palladium and platinum. Inorg Synth. 28, 60-63 (1990).
- Nataro, C., Fosbenner, S. M. Synthesis and Characterization of Transition-Metal Complexes Containing 1,1'-Bis(diphenylphosphino)ferrocene. J. Chem. Ed. 86 (12), 1412-1415 (2009).
- Kauffman, G. B., Pinnell, R. P. Copper (I) Iodide. Inorg. Synth. 6, 3-6 (1960).
- Sonogashira, K. J. Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. Organomet. Chem. 653 (1-2), 46-49 (2002).
- Chinchilla, R., Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 40 (10), 5084-5121 (2011).
- Kolb, H. C., Finn, M. G., Sharpless, K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 40 (11), 2004-2021 (2001).
- Spiteri, C., Moses, J. E. Copper-Catalyzed Azide-Alkyne Cycloaddition: Regioselective Synthesis of 1,4,5-Trisubstituted 1,2,3-Triazoles. Angew. Chem. Int. Ed. 49 (1), 31-33 (2010).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone