Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Pretreatment of Lignocellulosic Biomass with Low-cost Ionic Liquids
W tym Artykule
Podsumowanie
The pretreatment of lignocellulosic biomass with protic low-cost ionic liquids is shown, resulting in a delignified cellulose-rich pulp and a purified lignin. The pulp gives rise to high glucose yields after enzymatic saccharification.
Streszczenie
A number of ionic liquids (ILs) with economically attractive production costs have recently received growing interest as media for the delignification of a variety of lignocellulosic feedstocks. Here we demonstrate the use of these low-cost protic ILs in the deconstruction of lignocellulosic biomass (Ionosolv pretreatment), yielding cellulose and a purified lignin. In the most generic process, the protic ionic liquid is synthesized by accurate combination of aqueous acid and amine base. The water content is adjusted subsequently. For the delignification, the biomass is placed into a vessel with IL solution at elevated temperatures to dissolve the lignin and hemicellulose, leaving a cellulose-rich pulp ready for saccharification (hydrolysis to fermentable sugars). The lignin is later precipitated from the IL by the addition of water and recovered as a solid. The removal of the added water regenerates the ionic liquid, which can be reused multiple times. This protocol is useful to investigate the significant potential of protic ILs for use in commercial biomass pretreatment/lignin fractionation for producing biofuels or renewable chemicals and materials.
Wprowadzenie
Meeting humanity's energy demand sustainably is one of the greatest challenges that our civilization faces. Energy use is predicted to double in the next 50 years, putting greater strain on fossil fuel resources. 1 The buildup of greenhouse gases (GHG) in the atmosphere through wide-spread fossil fuel use is particularly problematic, as CO2 generated from combustion of fossil fuels is responsible for 50% of the anthropogenic greenhouse effect. 2 Therefore, large-scale application of renewable and carbon neutral technologies is essential for meeting the increased energy and material needs of future generations. 1, 3
Plant biomass is the most versatile renewable resource, as it can be used to produce heat, electricity as well as carbon-based chemicals, materials and fuels. Primary advantages of lignocellulosic biomass over other biomass types are its abundance, potential for high yields per area of land and often much higher CO2 emission savings, which includes high retention of carbon in the soil. 4, 5 Additional benefits of using biomass include local availability, low capital requirements to convert biomass to energy, and soil erosion prevention. 8
Major producers of lignocellulosic feedstocks are the forestry industry and the agricultural sector as well as municipal waste management. 6 Lignocellulose production has the potential to be expanded, with a mind to limiting deforestation and avoiding the replacement of food crops and release of potential pollutants. 7 For renewable biomass to become a viable widespread source of liquid transportation fuels and chemicals, its processing must become economically competitive with fossil fuel conversion technologies. 9, 10 A key to achieving this is to boost the yield and quality of biomass-derived intermediates while reducing cost.
Lignocellulose contains a high proportion of sugars which can be converted to fuels and chemicals via catalytic and microbial conversions. 11 These sugars are present in lignocellulose in polymeric form as cellulose and hemicellulose. They can be hydrolyzed into glucose and other sugar monomers and then used for producing bioethanol and other bio-derived chemicals and solvents. 12
In order to access the cellulosic sugars, pretreatment of the biomass is necessary through physical, chemical, or combined processes. 4 The pretreatment is arguably the most costly step in the valorization of lignocellulosic biomass. Hence research into improved pretreatment processes is imperative.
Various pretreatment technologies are available. Of particular interest are those that separate the lignin from cellulose (fractionative pretreatment). Lignin, the third major component in lignocellulose, limits access of hydrolyzing agents to cellulose and hemicellulose and reduces the sugar yield per ton of feedstock. 11 The separated lignin can be utilized as an additional biorefinery intermediate if it is isolated in suitable quality. 13 One fractionative process is the Kraft process which is the most common pretreatment for paper/cellulose production. In Kraft pulping, wood chips are placed in a mixture of sodium hydroxide and sodium sulfide and heated at elevated temperatures of around 170 °C under high pressure. 14 The alkaline reactions remove hemicellulose and lignin by breaking the polymers down to short fragments via nucleophilic and base catalysis, and by dissolving the lignin fragments via de-protonation of phenolic hydroxyl/alcohol groups. Another common delignification process is the Organosolv process which also fragments and dissolves the lignin and hemicellulose. Rather than using an alkaline aqueous solution, organic solvents such as ethanol and acetic acid are used at high temperatures ranging between 160-200 °C and pressures from 5-30 bar. Organosolv pretreatment has some advantages over Kraft pulping in that it produces less air and water pollution. 15 Both processes possess some economic challenges, if used for production of chemicals and fuels rather than cellulose. 16 The Ionosolv pretreatment uses ionic liquids, which are salts that have melting points below 100 °C and, as a result of their powerful Coulombic interactions, very low vapor pressures. 17 This eliminates air pollution in the pretreatment process, and enables processing at or near atmospheric pressure.
While most ILs are created in laborious, multi-step syntheses, protic ILs can be synthesized in a one-step process from commodity chemicals, which makes them less expensive; it is estimated that some ILs could be produced at bulk scale for a price of $1.24 per kg which is comparable to common organic solvents such as acetone and toluene. 18 The ability to recycle and reuse these customizable ILs in a process that operates at comparatively lower temperatures and pressures makes this a more benign alternative and an economically attractive candidate for biorefining.
This detailed video protocol demonstrates a lab-scale version of the Ionosolv process for the delignification of lignocellulosic biomass and the eventual enzymatic saccharification of the cellulose-rich pulp as well as the recovery of a high-purity odor-free lignin.19
Protokół
Note: The protic ionic liquids used in the process are synthesized in our laboratory, although some might be or become commercially available. The resulting ionic liquids are acidic and corrosive and probably skin/eye irritants (depending on the amine used), and must therefore be handled with care wearing appropriate PPE (lab coat, safety specs, resistant gloves).
1. Preparation
- Preparing and storing the lignocellulosic biomass
- Obtain the lignocellulosic biomass prior to the experiment in sufficient quantities, for example 100 g up to 5 kg.
Note: Each experiment requires at least 3 g of biomass (1 g each in triplicate). - Reduce the moisture content shortly after harvest and store the biomass in air-dried form in the laboratory. Air-dry the biomass by spreading the biomass on a table or bench and leaving it for 2 weeks or until it appears dry. Move and turn the biomass during this time to accelerate the process. Perform the fractionation experiments within a year of harvesting.
Note: Directly after harvesting, woody biomass can contain up to 50 wt% moisture, while air-dried biomass, which is more stable, contains 5-12 wt% moisture. - Grind and sieve the biomass to a select particle size range. Store the dried biomass in plastic bags or other suitable containers until use.
Note: For the small samples handled in this protocol, a reduced particle size is recommended, for example 180-850 µm.
- Obtain the lignocellulosic biomass prior to the experiment in sufficient quantities, for example 100 g up to 5 kg.
- Determining the moisture content of the biomass (according to the NREL protocol)20
- To determine the moisture content of the biomass, preweigh a piece of aluminum foil (size approximately 5 cm x 5 cm) on an analytical balance and record the weight of the foil (mfoil). Weigh out roughly 100 mg of air dried biomass onto the aluminum foil and record the exact air-dried weight (mADW).
- Fold the aluminum foil to make a packet and place in a fan-assisted oven at 105 °C overnight (at least for 4 hr).
- Take the packet out and place it immediately in a desiccator for 5 minutes, then weigh the packet immediately and record the exact weight of oven-dried weight plus foil (mODW+foil). Calculate the moisture content (in %) of the biomass mcBM according to equation 1:
 Eq. 1
Eq. 1
Where mODW+foil is the weight of the oven-dried packet (oven-dried biomass plus foil), mfoil is the weight of the foil and mADW is the air-dried weight of the biomass. All weights should be either in g or in mg.
- Synthesis of Ionic Liquid
- In a fume hood or vented enclosure, weigh 1 mole of the amine (triethylamine) into a 1 L round bottom flask with magnetic stir bar. Place the flask in an ice bath on a magnetic stirrer plate. Add a 250 ml addition funnel immediately to minimize evaporation of the amine.
Note: Ensuring the correct acid:base ratio is of high importance for achieving reproducibility of the pretreatment experiments. - Measure out 1 mole of sulfuric acid using a solution of known concentration (in this example 5 mol/L) and a volumetric flask (200 ml). Transfer the sulfuric acid into the addition funnel and rinse any acid adhering to the walls of the volumetric flask into the addition funnel with deionized water.
- Add the sulfuric acid dropwise to the amine while stirring vigorously. Make sure the solution does not heat up, as this would lead to boiling of the amine and an inaccurate ratio of acid to base. Rinse the inside of the addition funnel using deionized water to ensure the acid is transferred quantitatively.
Note: Larger batches of ionic liquid solution may be made by increasing the amount of amine and sulfuric acid as well as the flask volume accordingly. - Evaporate most of the water using a rotary evaporator. The water content should be lower than the water content required for the pretreatment.
Note: It is not necessary to completely dry the ionic liquid. It may be beneficial to leave some water in the ionic liquid, as some dried ionic liquids, including triethylammonium hydrogen sulfate, are solids at room temperature. A freeze dryer can also be used to lower the water content.
- In a fume hood or vented enclosure, weigh 1 mole of the amine (triethylamine) into a 1 L round bottom flask with magnetic stir bar. Place the flask in an ice bath on a magnetic stirrer plate. Add a 250 ml addition funnel immediately to minimize evaporation of the amine.
- Confirming and adjusting the Water Content of the IL solution
Note: The water content is an important experimental variable. There are three sources that the water in the pretreatment mixture may come from. All of them need to be taken into account: (1) the water contained in the synthesized or bought ionic liquid solution (2) the water contained in the air-dried biomass and (3) any water added with a pipette to achieve the final desired water content.- Determine the water content of the synthesized or purchased ionic liquid solution by volumetric Karl Fischer titration according to instructions issued by the titrator manufacturer. Add a few drops of the IL into the titrator using a pre-weighed syringe. Enter the weight of liquid added into the titrator and wait until the titrator displays a reading. Record the water content.
- Decide on a water content for the biomass pretreatment. In this experiment, use 20 wt%. Reduce the water content 5 wt% below the desired water content using a rotary evaporator and confirm the new water content by Karl Fischer titration as described in 1.4.1.
Note: Good results are obtained with 20 wt% water; however this may not always be the optimum for pretreatment. A higher water content may be chosen in order to reduce solvent cost or to lower viscosity.
- Calculations for the experiment.
- Decide on the amount of ionic liquid solution msol,final and a biomass-to-solvent ratio BM/solfinal. Here, use 10 g of ionic liquid solution containing 20 wt% water and a biomass-to-solvent ratio of 1:10 g/g.
Note: The tubes used in this protocol can fit up to 18 g of IL solution if the biomass to solvent ratio is 1:10 (wt/wt). A high biomass-to-solvent ratio (up to 1:2 or even 1:1) is favorable from an economic point of view but might compromise the pretreatment efficacy at small scale. - Determine the amount of (water-free) ionic liquid required for each sample according to the following equation 2:
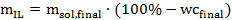 Eq. 2
Eq. 2
Where mIL is the required amount of ionic liquid in grams, msol,final is the desired amount of ionic liquid solution in grams, and wcfinal is the desired water content (in %) in the ionic liquid solution. - Next, calculate the amount of synthesized or bought ionic liquid solution to be added into each pressure tube according to the following equation 3:
 Eq. 3
Eq. 3
Where msol is the amount of solution to be added into each pressure tube (in grams), mIL is the required amount of ionic liquid (in grams), and wcsol is the water content in the ionic liquid solution (in %) as determined by Karl Fischer titration. - Determine the amount of biomass (oven-dried weight basis) to be added to the ionic liquid solution using equation 4 below. In this experiment, use 1 g of oven-dried Miscanthus biomass per pressure tube.
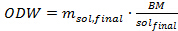 Eq. 4
Eq. 4
Where ODW is the amount of biomass to be added into each pressure tube (in grams), msol,final is the desired amount of ionic liquid solution (in grams) and BM/solfinal is the desired ratio of biomass-to-ionic liquid solution. - Determine the weight of air-dried biomass that needs to be added into the tube by using the following equation 5:
 Eq. 5
Eq. 5
where ADW is the air-dried weight of the biomass to be added into the tube (in grams), ODW is oven-dried weight of the biomass required for the experiment (determined in 1.5.4 in grams) and mcBM is the value determined in 1.2.3. - Calculate how much water needs to be added with a pipette to achieve the desired final water content according to the following equation 6:
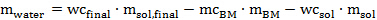 Eq. 6
Eq. 6
Where mwater is the amount of water to be added, wcfinal is the desired water content in the pretreatment mixture (here 20 wt%), msol.final is the amount of solvent (here 10 g), mcBM is the moisture content of the air-dried biomass, mBM is the amount of air-dried biomass to be added, wcsol is the water content of the synthesized or purchased ionic liquid solution, and msol is the amount of ionic liquid solution.
- Decide on the amount of ionic liquid solution msol,final and a biomass-to-solvent ratio BM/solfinal. Here, use 10 g of ionic liquid solution containing 20 wt% water and a biomass-to-solvent ratio of 1:10 g/g.
2. Pretreatment
Note: The process may be interrupted at any point by leaving the samples at room temperature (for a few days) or in the refrigerator (for longer periods).
- Pre-weigh three 15 ml pressure tubes with Teflon caps and silicone O rings. Visually inspect the pressure tubes to ensure they have no cracks or flaws.
Note: Larger pressure tubes fitting more ionic liquid and biomass can be used if desired, although pretreatment results will not be directly comparable between samples treated in different vial sizes. We recommend the use of front-sealing caps for better sealing. - With a 10 ml pipette, add the required amount of ionic liquid solution into the pressure tube standing on the scale. Use cork rings to keep the tube standing up. Record the weight of the ionic liquid solution added. Add the required amount of water to the solution determined in 1.5.6 using a pipette, assuming the density of water to be 1 g/ml.
- Add the required amount of air-dried lignocellulose by placing a piece of aluminum foil (dimensions 3 cm x 8 cm) on a balance, taring the balance and weighing out the biomass. Tare the balance and add the biomass to the tube. Place the empty foil back on the balance and record the difference.
Note: Alternatively, antistatic weighing boats may be used. - Close the lid with a Teflon cap with silicone O-ring. Check for a good seal without over-tightening. Record the weight of the pressure tubes containing biomass and ionic liquid. Mix the contents of the tube by using a vortex shaker until all of the biomass is in contact with the ionic liquid.
Note: Not all O-ring materials withstand being in contact with ionic liquids at elevated temperature. We found that silicone works well. - Place the pressure tubes in a fan-assisted oven that has been pre-heated to the desired temperature. For example, leave the tubes for 8 hr at 120 °C or for 1 hr at 150 °C.
Note: Time and temperature are important experimental variables. Other time – temperature combinations can be used. - Remove the vials from the oven using an oven glove and place them in a fume hood to let them cool to room temperature. Check the weight of the vials after cooling to assure no water escaped during cooking.
3. Pulp Wash
- Transfer the contents of each tube into a 50 ml centrifuge tube using 40 ml of absolute ethanol. Shake the tube using a vortex shaker for 1 min to mix well and leave the tube at room temperature for at least 1 hr.
Note: The separation can also be carried out using filtration, however, less accuracy may be observed for the suggested sample size. - Shake the tube using a vortex shaker for another 30 sec, then centrifuge the tube for 50 min at 2,000 x g. Separate liquid and solid by careful decanting. Collect the liquid in a clean 250 ml round bottom flask with stir bar.
- Add 40 ml fresh ethanol into the 50 ml tube and repeat step 3.2 three times.
- Remove the ethanol from the ionic liquid by placing the round bottomed flasks on a heating block. Connect each of them to a vacuum pump with a cold trap. Fill the trap with dry ice and set the heating to 40 °C. Switch on stirring and pump.
Note: This experiment uses a home-made parallel evaporator set-up based on a parallel synthesis set-up. Parallel evaporators can also be purchased ready-made. Alternatively a rotary evaporator can be used.
- Remove the ethanol from the ionic liquid by placing the round bottomed flasks on a heating block. Connect each of them to a vacuum pump with a cold trap. Fill the trap with dry ice and set the heating to 40 °C. Switch on stirring and pump.
4. Soxhlet Extraction of Pulp
- Transfer the wet washed pulp into cellulose thimbles and label each thimble using a pencil.
- Fill 150 ml absolute ethanol into a clean 250 ml round-bottomed flask with stir bar. Insert the sample-containing thimble into a 40 ml Soxhlet extractor, add a condenser and install everything on the parallel extractor work station connected to a recirculating chiller.
Note: If ethanol was used to transfer the pulp, add only the difference to 150 ml to the round-bottomed flask. - When all samples are loaded, begin stirring, set the temperature to 135 °C and turn on the recirculator (temperature setting 18 °C). Extract the pulp samples for 20 hr in total.
- Turn off the heating to allow refluxing to come to a halt. Then switch both stirring and cooling off. Take the thimbles out of the Soxhlet extractor using tweezers and let the wet pulp dry in the thimble overnight in a fume hood.
- Add the liquid from the Soxhlet extraction to the liquid from the biomass wash and continue evaporating the ethanol from the biomass wash with the parallel evaporator or a rotary evaporator at 40 °C.
- Transfer the air-dried pulp from the thimble onto a piece of tared aluminum foil on an analytical balance, record the air-dried weight of the extracted pulp and transfer it into a labelled plastic bag. Try to recover everything while not scraping thimble material off the wall.
- Determine the moisture content of the pulp immediately to calculate the oven-dried yield (as shown previously in step 1.4).
5. Lignin Isolation
- When all ethanol has evaporated, precipitate the lignin by transferring the ionic liquid from the round-bottom flask into a 50 ml centrifuge tube using 30 ml of water. Mix the suspension and leave for at least 1 hr. Centrifuge for 20 min at 2,000 x g, and separate the solution from the solid by decanting.
Note: In this protocol, 3 equivalents of water are used as antisolvent. Less antisolvent can be used, if desired. The washes can be collected, the water removed and the ionic liquid recovered for repeat use. - Add 30 ml of distilled water to the lignin pellet inside the centrifuge tube. Repeat the mixing, incubation for 1 hr and centrifugation. Repeat this step for a total of 3 lignin washes.
- Dry the lignin inside the centrifuge tube using a vacuum oven and a pierced lid at 45 °C overnight. To determine the lignin yield, place a piece of aluminum foil on the balance, tare the weight, add lignin from the oven and record the weight immediately. Transfer the lignin into a vial for storage.
Wyniki
The exact amount of lignin removal and lignin precipitation, recovered pulp and glucose yield depend on the type of biomass used, the temperature at which the treatment is run and the duration of the treatment. Short pretreatment times and low temperatures lead to incomplete pretreatment while at higher temperatures the cellulose becomes unstable in the ionic liquid, leading to hydrolysis and degradation. The selected ionic liquid also plays an important role in the outcome of the fractio...
Dyskusje
The technique for the fractionation of lignocellulosic biomass presented here produces a cellulose-rich pulp and a lignin. Most of the hemicelluloses are dissolved into the ionic liquid and hydrolyzed, but not recovered. If hemicellulose sugars are desired, a hemicellulose pre-extraction step prior to the Ionosolv delignification may be necessary. It has so far been impossible to fully close the mass balance for the biomass, as it is not possible to identify and quantify all degradation products found in the ionic liquid...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors acknowledge the Grantham Institute for Climate Change and the Environment, Climate-KIC and EPSRC (EP/K038648/1 and EP/K014676/1) for funding and Pierre Bouvier for providing experimental data for pine pretreatments.
Materiały
| Name | Company | Catalog Number | Comments | |
| IL synthesis | ||||
| Round bottom flask, with standard ground joint 24/29 NS, 1000 ml | Lenz | 3 0024 70 | VWR product code 271-1309 | |
| 250mL Addition Funnel, Graduated, 29/26 Joint Size, 0-4mm PTFE Valve | GPE | CG-1714-16 | ||
| Dish-shaped dewar flask, SCH 31 CAL | KGW-Isotherm | 1197 | ||
| Volumetric flask, 200 ml | VWR | 612-3745 | ||
| Cork rings, pasteur pipettes and teet, wash bottle with deionised water, large magentic stir bar | ||||
| Biomass size reduction | ||||
| Heavy Duty Cutting Mill SM2000 | Retsch | Discontinued | Replaced with Cutting Mill SM 200 (20.728.0001) | |
| Bottom sieves (10 mesh square holes, for particle size <2 mm) | Retsch | 03.647.0318 | Part of cutting mill | |
| Analytical Sieve Shaker AS 200 | Retsch | 30.018.0001 | Part of sieving machine | |
| Test Sieve 200 mm Ø x 50 mm height ISO 3310/1 (180 µm) | Retsch | 60.131.000180 | Part of sieving machine | |
| Test Sieve 200 mm Ø x 50 mm height ISO 3310/1 (850 µm) | Retsch | 60.131.000850 | Part of sieving machine | |
| Collecting pan, stainless steel, 200 mm Ø, height 50 mm | Retsch | 69.720.0050 | Part of sieving machine | |
| Rotary evaporator: | ||||
| Rotary evaporator (Rotavapor R-210) | Buchi | Discontinued | Replaced with Rotavapor R-300 | |
| Water bath (Heating bath B-491) | Buchi | 48201 | Part of rotary evaporator | |
| Recirculator | Julabo | F25 | Part of rotary evaporator | |
| Vacuum pump (MPC 101 Z) | Ilmvac GmbH | 412522 | Part of rotary evaporator | |
| Vacuum controller (Vacuum Control Box VCB 521) | Ilmvac GmbH | 600053 | Part of rotary evaporator | |
| Parallel evaporator: | ||||
| StarFish Base Plate 135mm (for Radleys & IKA) | Radleys | RR95010 | Part of parallel evaporator | |
| Monoblock for 5 x 250ml Flasks | Radleys | RR95130 | Part of parallel evaporator | |
| Telescopic 5-way Clamp with Velcro | Radleys | RR95400 | Part of parallel evaporator | |
| Gas/Vacuum Manifold with connectors | Radleys | RR95510 | Part of parallel evaporator | |
| 650mm Rod | Radleys | RR95665 | Part of parallel evaporator | |
| Quick Release Male, R/A Barbed 6.4mm + Shut-off (3.2mm ID) | Radleys | RR95520 | Part of parallel evaporator | |
| Stirrer/hot plate | Radleys | RR98072 | Part of soxhlet extractor | |
| Temperature controller | Radleys | RR98073 | Part of soxhlet extractor | |
| Elliptical Stirring Bar 15mm Rare Earth | Radleys | RR98097 | Part of parallel evaporator | |
| Vacuum cold trap, plastic coated, PTFE stopcock | Chemglass | CG-4519-01 | Part of parallel evaporator | |
| Vacuum pump (MPC 101 Z) | Ilmvac GmbH | 412522 | Part of parallel evaporator | |
| Tygon tubing E-3603, 6,40 mm (internal) 12,80 mm (external) | Saint-Gobain/VWR | 228-1292 | Part of parallel evaporator | |
| Parallel Soxhlet extractor: | ||||
| StarFish Base Plate 135mm (for Radleys & IKA) | Radleys | RR95010 | Part of soxhlet extractor | |
| Monoblock for 5 x 250ml Flasks | Radleys | RR95130 | Part of soxhlet extractor | |
| Telescopic 5-way Clamp with Velcro | Radleys | RR95400 | Part of soxhlet extractor | |
| Telescopic 5-way Clamp with Silicone Strap and Long Handle | Radleys | RR95410 | Part of soxhlet extractor | |
| Water Manifold with connectors | Radleys | RR95500 | Part of soxhlet extractor | |
| 650mm Rod | Radleys | RR95665 | Part of soxhlet extractor | |
| Quick Release Male, R/A Barbed 6.4mm + Shut-off (3.2mm ID) | Radleys | RR95520 | Part of soxhlet extractor | |
| Coil condensers with standard ground joints 29/32 NS | Lenz | 5.2503.04 | Part of soxhlet extractor | |
| Extractor Soxhlet 40mL borosilicate glass 29/32 socket 24/29 cone | Quickfit | EX5/43 | Part of soxhlet extractor | |
| Stirrer/hot plate | Radleys | RR98072 | Part of soxhlet extractor | |
| Temperature controller | Radleys | RR98073 | Part of soxhlet extractor | |
| Recirculator | Grant | LTC1 | Part of soxhlet extractor | |
| Cellulose extraction thimble | Whatman | 2280-228 | ||
| Tweezers | Excelta | 20A-S-SE | ||
| Vacuum drying oven: | ||||
| Vacuum drying oven | Binder | VD 23 | Part of vacuum oven | |
| Dewar vessel 2L 100x290mm with handle | KGW-Isotherm | 10613 | Part of vacuum oven | |
| Vacuum Trap | GPE | CG-4532-01 | Part of vacuum oven | |
| Other equipment: | ||||
| Analytical balance | A&D | GH-252 | accuracy to ± 0.1 mg | |
| Volumetric Karl Fischer titrator | Mettler Toledo | V20 | ||
| 10 mL disposable pipette | Corning Inc | Costar 4101 10 mL Stripette | ||
| Eppendorf Research plus pipette, variable volume, volume 100-1000 μL | Eppendorf | 3120000062 | ||
| Desiccator | Jencons | JENC250-028BOM | ||
| Ace pressure tube bushing type, Front seal, volume 15 mL | Ace Glass | 8648-04 | ||
| Ace O-rings, silicone, 2.6 mm, I.D. 9.2 mm | Ace Glass | 7855216 | O-ring for pressure tube | |
| Vortex shaker | VWR International | 444-1378 (UK) | ||
| Fan-assisted convection oven | ThermoScientific | HeraTherm OMH60 | ||
| Oven glove (Crusader Flex) | Ansel Edmont | 42-325 | ||
| 250 mL Round bottom flask single neck ground joint 24/29 (Pyrex) | Quickfit | FR250/3S | ||
| Rotaflo stopcock adapter with cone 24/29 | Rotaflo England | MF11/2/SC | ||
| 50 mL Falcon tube | Heraeus/Kendro | HERA 76002844 | ||
| Centrifuge (Mega Star 3.0) | VWR | 521-1751 | ||
| Reagents: | ||||
| Ethanol absolute | VWR | 20820.464 | ||
| Triethylamine | Sigma-Aldrich | T0886 | ||
| Sulfuric acid 5 mol/l (10N) AVS TITRINORM volumetric solution Safe-break bottle 2,5L | VWR | 191665V | ||
| Purified water (15 MΩ ressitance) | Elga | CENTRA R200 | ||
| Lignocellulosic biomass: | ||||
| Miscanthus X gigantheus | ||||
| Pinus sylvestris |
Odniesienia
- Lewis, N. S., Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc.Natl.Acad.Sci.U.S.A. 103 (43), 15729-15735 (2006).
- Dincer, I. Renewable energy and sustainable development: a crucial review. Renewable and Sustainable Energy Reviews. 4 (2), 157-175 (2000).
- Zweibel, K., Mason, J., Fthenakis, V. A solar grand plan. Sci. Am. 298 (1), 64-73 (2008).
- Lee, J. Biological conversion of lignocellulosic biomass to ethanol. J. Biotechnol. 56 (1), 1-24 (1997).
- Carrott, P., Ribeiro Carrott, M. Lignin-from natural adsorbent to activated carbon: A review. Bioresour.Technol. 98 (12), 2301-2312 (2007).
- Cardona Alzate, C., Sánchez Toro, O. Energy consumption analysis of integrated flowsheets for production of fuel ethanol from lignocellulosic biomass. Energy. 31 (13), 2447-2459 (2006).
- Field, C. B., Campbell, J. E., Lobell, D. B. Biomass energy: the scale of the potential resource. Trends Biochem Sci. 23 (2), 65-72 (2008).
- Hoogwijk, M., et al. Exploration of the ranges of the global potential of biomass for energy. Biomass Bioenergy. 25 (2), 119-133 (2003).
- Goldemberg, J. Ethanol for a sustainable energy future. Science. 315 (5813), 808-810 (2007).
- Himmel, M. E., et al. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 315 (5813), 804-807 (2007).
- Mosier, N., et al. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour.Technol. 96 (6), 673-686 (2005).
- Kumar, P., Barrett, D. M., Delwiche, M. J., Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. 48 (8), 3713-3729 (2009).
- Hu, F., Ragauskas, A. Suppression of pseudo-lignin formation under dilute acid pretreatment conditions. RSC Advances. 4 (9), 4317-4323 (2014).
- Chakar, F. S., Ragauskas, A. J. Review of current and future softwood kraft lignin process chemistry. Ind Crop Prod. 20 (2), 131-141 (2004).
- Mutjé, P., Pelach, M., Vilaseca, F., García, J., Jiménez, L. A comparative study of the effect of refining on organosolv pulp from olive trimmings and kraft pulp from eucalyptus wood. Bioresour.Technol. 96 (10), 1125-1129 (2005).
- Zhao, X., Cheng, K., Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 82 (5), 815-827 (2009).
- Brandt, A., Gräsvik, J., Hallett, J. P., Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 15, 550 (2012).
- Chen, L., et al. Inexpensive ionic liquids:[HSO 4]−-based solvent production at bulk scale). Green Chem. 16 (6), 3098-3106 (2014).
- Brandt, A., Chen, L., van Dongen, B. E., Welton, T., Hallett, J. P. Structural changes in lignins isolated using an acidic ionic liquid water mixture. Green Chem. 17, 5019-5034 (2015).
- Sluiter, A., et al. NREL/TP-510-42621. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples. , (2008).
- Sluiter, A., et al. NREL/ TP - 510 - 42618Determination of Structural Carbohydrates and Lignin in Biomass. Determination of Structural Carbohydrates and Lignin in Biomass. , (2011).
- Resch, M. G., Baker, S. R., Decker, NREL/TP-5100-63351. Low Solids Enzymatic Saccharificatin of Lignocellulosic Biomass. , (2015).
- Brandt, A., Ray, M. J., To, T. Q., Leak, D. J., Murphy, R. J., Welton, T. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid-water mixtures. Green Chem. 13 (9), 2489-2499 (2011).
- Aver, K., Scortegagna, A., Fontana, R., Camassola, M. Saccharification of ionic-liquid-pretreated sugar cane bagasse using Penicillium echinulatum enzymes. J Taiwan Inst Chem Eng. 45 (5), 2060-2067 (2014).
- George, A., et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 17 (3), 1728 (2015).
- Verdía, P., Brandt, A., Hallett, J. P., Ray, M. J., Welton, T. Fractionation of lignocellulosic biomass with the ionic liquid 1-butylimidazolium hydrogen sulfate. Green Chem. 16 (3), 1617-1627 (2014).
- Brandt, A., et al. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid-water mixtures. Green Chem. 13 (9), 2489-2499 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone