Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Recording Spatially Restricted Oscillations in the Hippocampus of Behaving Mice
W tym Artykule
Podsumowanie
This protocol describes the recording of local field potentials with multi-shank linear silicon probes. Conversion of the signals using current source density analysis allows the reconstruction of local electrical activity in the mouse hippocampus. With this technique, spatially restricted brain oscillations can be studied in freely moving mice.
Streszczenie
The local field potential (LFP) emerges from ion movements across neural membranes. Since the voltage recorded by LFP electrodes reflects the summed electrical field of a large volume of brain tissue, extracting information about local activity is challenging. Studying neuronal microcircuits, however, requires a reliable distinction between truly local events and volume-conducted signals originating in distant brain areas. Current source density (CSD) analysis offers a solution for this problem by providing information about current sinks and sources in the vicinity of the electrodes. In brain areas with laminar cytoarchitecture such as the hippocampus, one-dimensional CSD can be obtained by estimating the second spatial derivative of the LFP. Here, we describe a method to record multilaminar LFPs using linear silicon probes implanted into the dorsal hippocampus. CSD traces are computed along individual shanks of the probe. This protocol thus describes a procedure to resolve spatially restricted neuronal network oscillations in the hippocampus of freely moving mice.
Wprowadzenie
Oscillations in the LFP are critically involved in information processing by neuronal circuits. They cover a wide spectrum of frequencies, ranging from slow waves (~1 Hz) to fast ripple oscillations (~200 Hz)1. Distinct frequency bands are associated with cognitive functions including memory, emotional processing, and navigation2,3,4,5,6,7. Current flow across neuronal membranes constitutes the largest part of the LFP signal8. Cations entering the cell (e.g. via activation of glutamatergic excitatory synapses) represent an active current sink (as charge leaves the extracellular medium). In contrast, net flow of positive charge to the extracellular medium, for instance by activation of GABAergic inhibitory synapses, depicts an active current source at that location. In neuronal dipoles, current sinks are paired with passive sources and vice versa due to compensating currents affecting membrane charge at distant sites.
The electrical field produced by remote neural processes can also result in considerable voltage deflections on a recording electrode and might thus be falsely considered as a local event. This volume conduction poses a serious challenge to the interpretation of LFP signals. CSD analysis provides information about local current sinks and sources underlying LFP signals and therefore comprises a means to reduce the impact of volume conduction8. In laminated structures like the hippocampus, one-dimensional CSD signals can be obtained by the second spatial derivative of the LFP recorded from equidistant electrodes arranged perpendicular to the laminar planes9. The advent of commercially available linear silicon probes has allowed researchers to utilize the CSD method for the study of local oscillation activity in the hippocampus. For example, it has been demonstrated that distinct gamma oscillations emerge in a layer-specific manner in the CA1 area10. Furthermore, CSD analysis has identified independent hot spots of gamma activity in the principal cell layer of the dentate gyrus11. Importantly, these findings were only apparent in local CSD but not in LFP signals. CSD analysis thus provides a powerful tool to gain insight in the microcircuit operations of the hippocampus.
In this protocol, we provide a comprehensive guide to obtain one-dimensional CSD signals with silicon probes. These methods will enable users to investigate localized oscillation events in the hippocampus of behaving mice.
Protokół
All methods involving living animals have been approved by the Regierungspräsidium Freiburg in accordance with the German Animal Welfare Act.
1. Preparations
- Design and build an appropriate insertion tool transiently carrying the silicon probe and the electrode connector during the process of implantation. See Figure 1 for an example custom built insertion tool.
- Carefully release the silicon probe and electrode connector from its packaging using ceramic-tipped forceps.
- Lift the connector board and securely fix it with a crocodile clamp attached to a stand.
- Using a stereoscope, align the probe with the insertion tool with ceramic-tipped forceps. Apply a ~2 mm layer of paraffin wax melted with a cauterizer to glue the probe to the insertion tool. Take care not to touch the probe shanks during this procedure.
- Fix the electrode connector to the shaft of the insertion tool using standard adhesive tape. Note that depending on the manufacturer, ground wires might need to be soldered to the electrode connector board prior to implantation. Remove the insulation from two short pieces of varnish-insulated copper wire using tin-solder applied with a soldering iron (400 °C). Solder the ground wires to the appropriate slots in the electrode connector board.
- Remove the insulation of two additional pieces of copper wire. Wrap each bare copper wire three times around a stainless steel screw (1 mm diameter, 2 mm length). Apply flux suitable for soldering steel and solder the copper wire to the bottom of the screw cap. Make sure that the bottom half of the screw thread remains free of tin-solder.
- Use a standard multimeter to check for electrical contact between wire and screw.
- Disinfect the shanks of the silicon probe and the ground screws by immersion in 70% ethanol (10 s).
- Prepare a protective cover for the probe implant by cutting the head of a plastic Pasteur pipette in half.
2. Implantation Surgery
- Sterilize surgical instruments (scissors, fine-tipped forceps, surgical clamps) with a hot bead sterilizer. Wipe all surfaces with 70% ethanol.
- Induce anesthesia with 3% isoflurane in oxygen delivered at ~1 L/min.
- For maintenance, use 1 - 1.5% isoflurane. Note that the isoflurane concentration required to achieve surgical tolerance can vary from animal to animal.
- Stable surgical tolerance is achieved when the animal fails to respond to toe-pinching. Monitor the breathing rate of the mouse and adjust the concentration of isoflurane if necessary.
- Apply ointment to the animal's eyes to prevent drying out.
- Mount the mouse in a stereotaxic frame by gently inserting ear bars into the ear canal. Once the head of the mouse is stabilized by the ear bars, place a mouth piece over the snout for continuous isoflurane delivery. Place the mouse on towel or pad over a heating pad and inject buprenorphine subcutaneously (0.05 - 0.1 mg/kg body weight) to ensure postoperative analgesia.
- Shave the head with a standard shaver and disinfect the skin with 70% ethanol. Using surgical scissors, make an incision into the skin along the midline of the skull and open the skin using surgical clamps.
- Align the head of the animal with the aid of a stereotaxic alignment tool to level bregma and lambda. There should be less than 50 µm of height offset between bregma and lambda. Furthermore, level the head along the mediolateral axis by measuring the depth from bregma at the skull surface at defined distances left and right (e.g., 1 mm left and right of bregma). Adjust the tilt of the head if necessary.
- Clean the head with 3% hydrogen peroxide and wipe dry with sterile cotton wipes.
- Determine the location of the craniotomy relative to bregma using an appropriate stereotaxic atlas12.
- Using a 0.9 mm drill head, drill two screw holes in the bone over the cerebellum to place ground and reference screws. Additionally, 1 - 3 holes for anchoring screws are desirable to stabilize the implant. The location of anchor screws will depend on the location of the craniotomy. For implantation into the hippocampus, place anchor screws over the contralateral parietal and ipsilateral frontal cortex. Insert the screws in the bone using a suitable screwdriver. Take care not to penetrate into the brain.
- Perform the craniotomy by slowly thinning the skull with the drill in a rectangular area around the implantation side. Frequently moisten the bone with sterilized phosphate buffer (PB). The remaining thinned skull can be gently pierced and removed with the aid of a fine (27G) injection needle and a pair of tweezers.
- Carefully pierce the dura mater with a thin (27G) injection needle. Form a small hook by bending the tip of the needle with a pair of tweezers and pull the dura for removal. Apply PB to prevent the brain surface from drying out.
- Mount the electrode insertion tool on a stereotaxic holder, zero the probe on bregma, and move the probe to the stereotaxic coordinates over the craniotomy. Slowly penetrate the brain surface. Make sure the probe shafts do not bend. Avoid implanting through blood vessels.
- Slowly lower the probe until ~200 µm above the desired depth. Cover the craniotomy and shanks of the silicon probe with sterilized Vaseline for protection. Apply dental cement to fix the base of the probe to the anchoring screw in the skull.
- Right after cement application, slowly move the probe to the target depth. Advancing the last ~200 µm after application of the cement reduces lateral movement of the probe and ensures minimal tissue damage in the target area. Note that the curing time of the cement used can affect this step of the protocol. With quickly curing cement, omit this step and directly implant the probe to the target depth in order to avoid damage to the silicon probe.
- After the cement has cured, release the probe from the insertion tool by melting the wax with a cauterizer.
- Release the connector board from the insertion device and position it at a suitable place on the skull using a crocodile clamp attached to the insertion handle. In case of probe implantation into the hippocampus, place the connector board on the contralateral parietal bone. Fix the connector board to the skull using dental cement.
- Solder the ground and reference wires of the connector board to the wires attached to the two screws over the cerebellum.
- Trim the protective cover to the correct height and place it over the silicon probe. Fix the cover to the connector board and skull using dental cement, avoiding the skin around the exposed skull. Suturing the skin around the implantation site is usually not required.
3. Recovery After Surgery
- Apply appropriate analgesic treatment for at least 2 days (e.g. subcutaneous injections of buprenorphine every 6 h during daytime and in the drinking water overnight combined with carprofen (4 - 5 mg/kg body weight) subcutaneously every 24 h). Single-housing is recommended to prevent damage to the implant.
- Allow at least one week for recovery. Consult with local animal welfare guidelines.
4. Data Acquisition
- Record LFPs from freely moving mice using a suitable data acquisition system connected through a commutator. To acquire LFPs, use a sampling frequency of 1 - 5 kHz. Higher sampling rates (20 - 30 kHz) are required if single-unit discharges are to be recorded along with the LFP.
- Store raw recording files of the individual channels for offline analysis.
5. Histology
- After completion of the recording, deeply anesthetize the animal (e.g. 2 g/kg body weight urethane injected intraperitoneally). Confirm the anesthetic state by lack of response to toe pinching.
- Perfuse the mouse transcardially with ice-cold phosphate-buffered saline (~1 min) followed by 4% paraformaldehyde (~10 min) using standard intracardial perfusion methods13. Before perfusion, electrolytic lesioning of the recording sites might by performed (e.g. by applying 10 - 20 V of constant voltage for up to 1 s). Alternatively, fluorescent dyes applied to the shank tips before implantation can be used to track identification. Testing the various methods for the identification of electrode positions to obtain optimal results with different types of silicon probes is recommended.
- Cut brain sections (~100 µm) and stain the slices with 4'-6-diamidino-2-phenylindole (DAPI, 1 µg/mL) followed by three washing steps in PB (each 10 min at room temperature).
- Place the sections on a microscope slide, apply a drop of embedding medium and cover the section with a cover slip. Let the embedding medium dry overnight at room temperature.
- Using an epifluorescence or confocal laser-scanning microscope, identify the location of the recording sites.
- To attempt recovery of the silicon probe for further use, hold the probe with a crocodile clamp and release the probe from the scull by carefully melting the dental cement with a soldering iron (400 °C). Take care not to touch the probe shanks during this procedure!
- Wash the probe in hot distilled water (~80 °C, 15 min) followed by enzymatic solution (1% Tergazyme in distilled water, 30 min at room temperature) and another washing step in distilled water (15 min). Note that the success rate of probe recovery is low.
6. CSD Analysis
- Using a suitable analysis environment (e.g. Python), convert LFP data of an individual shank to CSD by approximating the second spatial derivative along the shank as
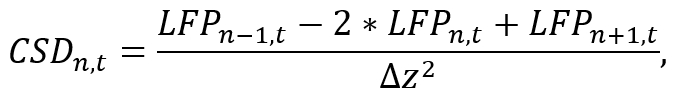
where LFPn,t is the LFP signal on the nth electrode at time t and Δz is the inter-electrode spacing. Note that due to the n-1 and n+1 operations, the CSD of the first and last electrodes of the shank cannot be estimated, which has to be taken under consideration during probe placement. Implement the approximation formula using a short segment of code that computes the CSD signal for each electrode while iterating over time (see Supplemental Code File). - Use the obtained CSD signal for further analysis (e.g., studying specific frequency bands of brain oscillations by applying band-pass filters).
Wyniki
Figure 1 illustrates the insertion tool used for the implantation of silicon probes. Recordings from chronically implanted silicon probes targeting the CA1 area and the granule cell layer of the dentate gyrus are shown in Figure 2. We recorded LFPs from the probe shanks during free movement in the homecage. To minimize the effect of volume conduction, the obtained signals were converted to CSD along each shank of the probe (
Dyskusje
Increasing evidences indicate that brain oscillations in hippocampal neuronal circuits occur in discrete spatial domains10,11,16. CSD analysis drastically reduces the influence of volume conduction, a crucial prerequisite for the study of local oscillation events. With this video, we provide a guide to implanting silicon probes into the mouse hippocampus for the analysis of CSD data. We show representative examples of CSD signal...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We are grateful to Karin Winterhalter and Kerstin Semmler for technical assistance. This work was supported by the cluster of excellence BrainLinks - BrainTools (EXC 1086) of the German Research Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| Crocodile clamp with stand | Reichelt Elektronik | HALTER ZD-10D | |
| Silicon probe | Cambridge Neurotech | P-series 32 | |
| Stereoscope | Olympus | SZ51 | |
| Varnish-insulated copper wire | Bürklin Elektronik | 89 F 232 | |
| Ground screws | Screws & More GmbH (screwsandmore.de) | DIN 84 A2 M1x2 | |
| Flux | Stannol | 114018 | |
| Ceramic-tipped forceps | Fine Science Tools | 11210-60 | |
| Paraffine Wax | Sigma-Aldrich | 327204 | |
| Cauterizer | Fine Science Tools | 18010-00 | |
| Soldering iron | Kurtz Ersa | OIC1300 | |
| Multimeter | Uni-T | UT61C | |
| Ethanol | Carl Roth | 9065.1 | |
| Pasteur pipettes | Carl Roth | EA65.1 | |
| Heat sterilizer | Fine Science Tools | 18000-45 | |
| Stereotaxic frame | David Kopf | Model 1900 | |
| Stereotaxic electrode holder | David Kopf | Model 1900 | |
| Isoflurane | Abbvie | B506 | |
| Oxygen concentrator | Respironix | 1020007 | |
| Buprenorphine | Indivior UK Limited | ||
| Electrical shaver | Tondeo | Eco-XS | |
| Heating pad | Thermolux | 463265/-67 | |
| Surgical clamps | Fine Science Tools | 18050-28 | |
| Hydrogen peroxide | Sigma-Aldrich | H1009 | |
| Sterile cotton wipes | Carl Roth | EH12.1 | |
| Drill | Proxxon | Micromot 230/E | |
| 21G injection needle | B. Braun | 4657527 | |
| Phosphate buffer/phosphate buffered saline | |||
| Stereotaxic atlas | Elsevier | 9.78012E+12 | |
| Surgical scissors | Fine Science Tools | 14094-11 | |
| Surgical forceps | Fine Science Tools | 11272-40 | |
| 27G injection needles | B. Braun | 4657705 | |
| Vaseline | |||
| Dental cement | Sun Medical | SuperBond T&M | |
| Carprofen | Zoetis | Rimadyl 50mg/ml | |
| Recording amplifier | Intan Technologies | C3323 | |
| USB acquisition board | Intan Technologies | C3004 | |
| Recording cables | Intan Technologies | C3216 | |
| Electrical commutator | Doric lenses | HRJ-OE_FC_12_HARW | |
| Acquisition software | OpenEphys (www.open-ephys.org) | GUI | allows platform-independent data acquisition |
| Computer for data acquisition | |||
| Analysis environment | Python (www.python.org) | allows platform-independent data analysis | |
| Urethane | Sigma-Aldrich | ||
| Vibratome | Leica | VT1000 | |
| Microscope slides | Carl Roth | H868.1 | |
| Cover slips | Carl Roth | H878.2 | |
| Embedding medium | Sigma-Aldrich | 81381-50G | |
| Distilled water | Millipore | Milli Q | Table-top machine for the production of distilled water |
| Tergazyme | Alconox | Tergazyme |
Odniesienia
- Buzsáki, G., Draguhn, A. Neuronal oscillations in cortical networks. Science. 304 (5679), 1926-1929 (2004).
- Keefe, J., Recce, M. L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 3 (3), 317-330 (1993).
- Benchenane, K., et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 66 (6), 921-936 (2010).
- Jadhav, S. P., Kemere, C., German, P. W., Frank, L. M. Awake hippocampal sharp-wave ripples support spatial memory. Science. 336 (6087), 1454-1458 (2012).
- Yamamoto, J., Suh, J., Takeuchi, D., Tonegawa, S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 157 (4), 845-857 (2014).
- Karalis, N., et al. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nature Neuroscience. 19 (4), 605-612 (2016).
- Khodagholy, D., Gelinas, J. N., Buzsáki, G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science. 358 (6361), 369-372 (2017).
- Buzsáki, G., Anastassiou, C. A., Koch, C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience. 13 (6), 407-420 (2012).
- Mitzdorf, U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiological Reviews. 65 (1), 37-100 (1985).
- Lasztóczi, B., Klausberger, T. Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron. 81 (5), 1126-1139 (2014).
- Strüber, M., Sauer, J. -. F., Jonas, P., Bartos, M. Distance-dependent inhibition facilitates focality of gamma oscillations in the dentate gyrus. Nature Communications. 8 (1), 758 (2017).
- Franklin, K. B. J., Paxinos, G. . The mouse brain in stereotaxic coordinates. , (2007).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. Journal of Visualized Experiments. (65), e3564 (2012).
- Kajikawa, Y., Schroeder, C. E. How local is the local field potential?. Neuron. 72 (5), 847-858 (2011).
- Berens, P., Keliris, G. A., Ecker, A. S., Logothetis, N. K., Tolias, A. S. Feature selectivity of the gamma-band of the local field potential in primate primary visual cortex. Frontiers in Neuroscience. 2 (2), 199-207 (2008).
- Lastóczi, B., Klausberger, T. Distinct gamma oscillations in the distal dendritic field of the dentate gyrus and the CA1 area of mouse hippocampus. Brain Structure and Function. 222 (7), 3355-3365 (2017).
- Nguyen Chi, V., Müller, C., Wolfenstetter, T., Yanovsky, Y., Draguhn, A., Tort, A. B. L., Brankačk, J. Hippocampal respiration-driven rhythm distinct from theta oscillations in awake mice. Journal of Neuroscience. 36 (1), 162-177 (2016).
- Chung, J., Sharif, F., Jung, D., Kim, S., Royer, S. Micro-drive and headgear for chronic implant and recovery of optoelectronic probes. Scientific Reports. 7 (1), 2773 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone