Method Article
Intraoperative Visualization of Subretinal Injection and Retinal Detachment in Rats
W tym Artykule
Podsumowanie
This article describes methods for subretinal injection in rats, utilizing intraoperative visualization to control both the injection site and the area of retinal detachment.
Streszczenie
Subretinal injection, the delivery of a solution between the photoreceptors and the retinal pigment epithelium (RPE), creates a subretinal space where components are in direct contact with photoreceptors and RPE cells. This delivery method allows for targeted treatment of these cells. Therapeutics for subretinal injection have been developed and approved, particularly for inherited retinal diseases. In animals, subretinal injection procedures can be challenging due to the lens size, especially in rodents. This article describes methods for subretinal injection in rats, enabling intraoperative visualization and control of both the injection site and the size of the detached area, as performed in humans. The procedure is conducted under general and local anesthesia and requires pupil dilation. Using an ophthalmic microscope, the subretinal injection is performed through a 30 G scleral channel, with the cannula tip gently applied to the retina to create a retinotomy. Volumes ranging from 10-25 µL can be delivered, corresponding to two-fifths to half of the rat retina. Immediate postoperative examinations using fundus photography and optical coherence tomography confirm successful delivery into the subretinal space with visible subretinal fluid. The major risks of this procedure include lens damage (cataract), detachment failure, intravitreal hemorrhage, subretinal hemorrhage, and postoperative keratitis. In addition to delivering therapeutics into the subretinal space, this technique is used to induce short-term or long-term retinal detachment using aqueous or viscous products, respectively. Unlike the transscleral approach, this method enables precise intraoperative positioning of the retinal detachment.
Wprowadzenie
The retina is a light-sensitive neuronal tissue located in the inner and posterior part of the eyeball. The external layer of the neuroretina contains photoreceptors, which are specialized neurons dedicated to converting light into electrochemical signals and are thus essential for vision. The retinal pigment epithelium (RPE), located beneath the neuroretina, provides extensive metabolic support to photoreceptors, facilitating visual pigment regeneration and the regular renewal of their outer segments1.
Photoreceptor degeneration is the most common cause of irreversible vision loss and occurs in various retinal diseases, such as age-related macular degeneration, retinitis pigmentosa, diabetic retinopathy, and retinal detachment1,2,3,4. Most of these conditions result from the primary or secondary loss of photoreceptors, often secondary to RPE loss or dysfunction. Therefore, it is particularly important to study photoreceptors and RPE cells in vivo to prevent their degeneration. To date, no effective therapies exist for numerous retinal diseases, such as retinal dystrophies (including retinitis pigmentosa) or macular degeneration (including age-related macular degeneration).
Subretinal injection, the delivery of a solution between the photoreceptors and the RPE, creates a subretinal space where components are in direct contact with photoreceptors and RPE cells. This delivery method allows for targeted treatment of these cells, bypassing various retinal or vascular barriers. Several therapeutics for subretinal delivery have been developed and approved, particularly for inherited retinal diseases5,6.
It is important to harmonize surgical practices for subretinal delivery. In humans, the standard procedure involves 25 G pars plana vitrectomy, which provides relatively easy access to the posterior segment of the eye because the lens is small in proportion to the entire eye. Subretinal delivery is performed through a 25 G trocar using a 38 G or 41 G silicone-tipped biocompatible cannula, allowing for smooth transretinal flow5.
In animals, subretinal injection procedures can be challenging due to the lens size. For instance, in rodents, the lens occupies up to 90% of the vitreous cavity. In rats, the lens takes up less space than in mice but remains significant (see Figure 1A). Additionally, subretinal injections can be used to model retinal detachment (RD) by using viscous solutions to prevent subretinal fluid clearance by RPE cells7,8. These models are primarily employed in the development of therapeutics for retinal diseases.
This article describes methods for subretinal delivery or induced RD in rats, enabling total intraoperative control of the injection site and the detached retinal area.
Protokół
Experiments and procedures were approved by the Local Animal Ethics Committee and conducted in facilities associated with the laboratory, in compliance with local legislation. All experimental work adhered to institutional policies on biosecurity and safety procedures (Local Animal Ethics Committee Charles Darwin CEEACD #5) and followed European Directive 2010/63/EU. Eight-week-old wild-type female Long Evans rats (Rattus norvegicus) were used in this study. Various retinal degeneration models of rats, such as P23H and RCS, can be used to test the subretinal delivery of different therapeutics, including drugs and gene therapies. The details of the reagents and equipment used in this study are provided in the Table of Materials.
1. Preparation of solutions
NOTE: To prevent surgical site infection or solution contamination, handle with pre-sterilized instruments and sterile solutions prepared in a fume cupboard.
- Subretinal delivery: Dilute molecules in PBS or dimethyl sulfoxide for aqueous subretinal delivery.
- Induction of RD: Use 2% hydroxypropyl methylcellulose or 1%-5% sodium hyaluronate to induce long-term RD.
2. Experimental setup
- Mount a nonbeveled 10 mm 30 G cannula metal tip cemented on a 10 µL or 25 µL syringe onto a microinjector.
- Load the prepared solution into the syringe.
3. Animal preparation
- Anesthesia
- General anesthesia: Anesthetize the rats with an intraperitoneal injection of 40 mg/kg ketamine and 0.14 mg/kg medetomidine.
NOTE: This anesthesia lasts long enough for the duration of the procedure and for immediate postoperative explorations. - Pupil dilation: Put one drop of 0.5% tropicamide to obtain mydriasis in one eye.
NOTE: For right-handed surgeons, the procedure will be easier in the left eye, and vice versa. - Local anesthesia ( Figure 2B): Put one eyedrop of oxybuprocaine in the eye to be operated.
NOTE: To ensure full effectiveness, wait 30 s after applying the local anesthetic before touching the eye.
- General anesthesia: Anesthetize the rats with an intraperitoneal injection of 40 mg/kg ketamine and 0.14 mg/kg medetomidine.
4. Surgery under the microscope
- Intraoperative visualization: Use an ophthalmic microscope connected to a foot switch to perform the surgery.
- Scleral exposure (Figure 2C,D)
- Use two eyelid pulling sutures (5-0 or 6-0) anchored at the external quarter of the palpebral margin at the upper and lower eyelids.
- Pull the sutures to achieve gentle bulging of the eye.
- Scleral channel (Figure 2E,F): Use a 30 G needle to create a scleral channel through the temporal bulbar conjunctiva, approximately 1-2 mm from the corneal limbus.
NOTE: Barely insert the beveled needle tip without pushing it further to avoid the risk of lens touch and subsequent cataract. Observe for conjunctival hemorrhage for tens of seconds at this stage. Avoid tamponading the bleeding, as this can cause blood to pool in the vitreous and jeopardize the procedure. Gently wipe away any blood in the channel area or that impedes visualization using a cotton swab. - Cornea protection (Figure 2G): Use a tear gel as a lens-eye interface throughout the procedure.
- Visualization of the retinal plane (Figure 2G)
- Place a flat contact lens (8 mm diameter) on the eye surface to visualize the retinal plane with proper magnification.
- Use the footswitch of the microscope to focus on the retinal plane.
- Subretinal injection (Figure 2H - M)
- Insert the cannula vertically through the preformed scleral channel (Figure 2H).
- Avoid touching the lens by keeping the cannula vertical.
- Slowly approach the retina and gently press it until retinal whitening is observed (arrows in Figure 2I).
- Maintain the tip's stability while injecting through the retinotomy formed by the injection flow (Figure 2J). Avoid withdrawing the tip too soon from the retinotomy, as this will cause the product to be injected into the vitreous.
- After the injection, remove the tip from the retinotomy, and then gently withdraw the entire cannula from the vitreous cavity, ensuring the lens is not touched (Figure 2K).
NOTE: If the bleb is insufficiently developed or the detached retinal area is too restricted once the syringe is empty, perform a reinjection using the same scleral channel and inserting the tip into the previously formed retinotomy. - Ensure the pupillary red reflex is altered by the retinal detachment without observing blood flow within the vitreous cavity (Figure 2L,M).
- End of the surgery (Figure 2N,O)
- Remove the contact lens, the eyelid sutures, and swab any bleeding carefully (Figure 2N).
- Briefly assess the intraocular pressure manually.
NOTE: A small amount of vitreous fluid may emerge into the subconjunctival space, but active leakage must not occur (Figure 2O).
5. Postoperative care and awakening
- Apply chloramphenicol-retinol eye ointment to the operated eye.
- Administer 1 mL of 5% glucose monohydrate intraperitoneally.
- Inject 0.9 mg/kg atipamezole subcutaneously.
- Place the animal in a temperature-controlled chamber and monitor it until it wakes up.
Wyniki
The success of a subretinal injection relies on precise and exclusive delivery into the subretinal space (i.e., between the photoreceptors and the RPE layers) through the retinotomy, avoiding subretinal hemorrhage, as blood is toxic to photoreceptors and RPE cells. Successful delivery is confirmed by intraoperative visualization (Figure 2L), immediate postoperative fundus photography, and optical coherence tomography (OCT). Fundus photography rules out subretinal hemorrhage, while OCT B-scans confirm retinal detachment (RD) with clear subretinal fluid (Figure 1 and Figure 3). Additionally, fundus photography can identify lens touch or induced cataracts that may be unnoticed during the procedure (Figure 3C,D). Both photography and OCT scans can estimate the size of the detached area or bleb (Figure 1B-E).
Several factors influence the size of the bleb: (1) Injection site location: The farther the injection site is from the optic disc, the smaller the detached area; (2) Injected volume: An injection volume of 20-25 µL can detach approximately half of the retina in rats; (3) Quality of the detached retina: In retinal degeneration models (e.g., P23H, RCS), detachment may be challenging due to increased resistance between photoreceptors and RPE cells caused by degenerative processes, such as gliosis and subretinal material accumulation. In such cases, induced retinal detachments are typically flat, and OCT scans are crucial for clearly delineating the detached area.
A minor intravitreal hemorrhage may occur at the injection site because retinal microvessels cannot always be completely avoided (Figure 3E). However, such hemorrhages typically do not extend into the subretinal space.
In cases of insufficient detachment, the issue must be recognized intraoperatively, necessitating immediate reinjection through the preformed retinotomy. During an experimental session with 20 rats, two surgeons (one experienced and one beginner) performed the step-by-step method. The experienced surgeon achieved success in 9 out of 10 procedures, while the beginner succeeded in 6 out of 10.
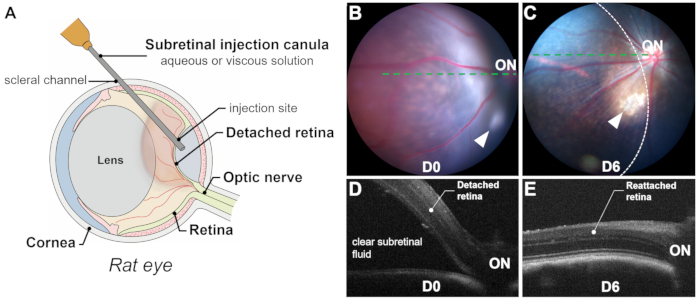
Figure 1: Overview of the process and results. (A) Schematic representation of the subretinal injection process in a rat eye, showing the significant volume of the lens. (B,C) Fundus photography immediately after the injection (B) and 6 days post-injection (C). Arrowheads indicate the injection site. The retinal detachment area is outlined by a dashed line. (D,E) Optical coherence tomography B-scans immediately after the injection (D) and 6 days post-injection (E).ON: optic nerve. Magnification: 1x. Please click here to view a larger version of this figure.
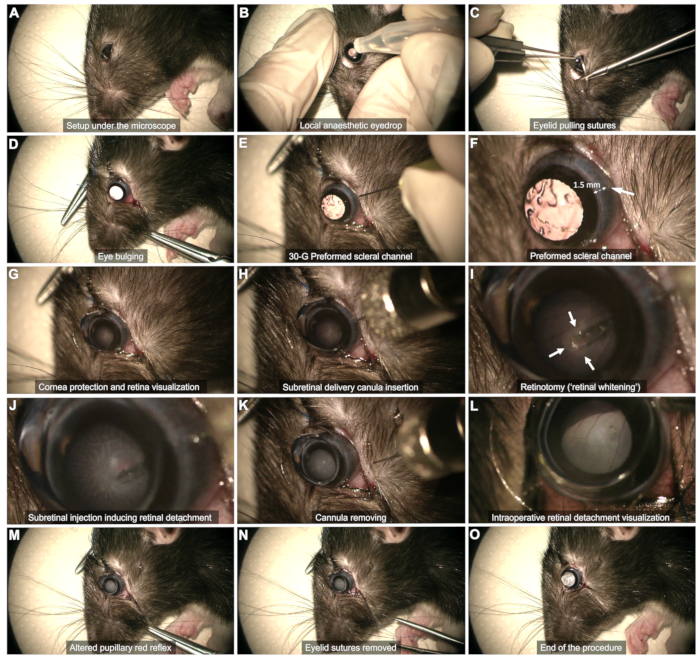
Figure 2: Steps of the Subretinal Injection Protocol. The protocol is demonstrated in an 8-week-old Long Evans rat under general anesthesia. For a detailed description of each step, refer to the video or the "Protocol" section in the text. Please click here to view a larger version of this figure.

Figure 3: Examples of various postoperative fundus photography outcomes. (A) Normal postoperative appearance with minor pigment dispersion at the injection site (arrow). (B) Normal postoperative appearance with a small intravitreal air bubble at the injection site. These small air bubbles originate from the syringe and are harmless, even in the subretinal space. (C,D) Lens touch resulting in minor (C) or significant (D) lens opacification (cataract). (E,F) Minor (E) or more extensive (F) intravitreal bleeding localized near the injection site, with no spread into the subretinal space. (G) Injection site bleeding spreading predominantly into the subretinal space. In this case, retinotomy caused an injury of a retinal blood vessel. (H) Extensive intravitreal hemorrhage resulting from scleral penetration. Magnification: 1x. Please click here to view a larger version of this figure.
Dyskusje
This article describes methods for transvitreal subretinal delivery in rats with intraoperative visualization and control of both the injection site and the detached area.
There are two critical steps in this protocol: scleral exposure and retinotomy. Proper positioning, exposure, and access are fundamental prerequisites for any surgical procedure. In this case, it is crucial to ensure optimal access to the sclera and the injection site. Insufficient scleral exposure can lead to avoidable difficulties in subsequent steps. Additionally, the quality of the retinotomy determines the risk of hemorrhage. It should be performed as gently as possible and as far as possible from any retinal vessels. If done correctly, the risk of significant intravitreal or subretinal bleeding is very low.
When using this approach for subretinal injection, retinal detachment (RD) induction may fail for several reasons. The most challenging step in the learning process is the injection itself. The injection site must be carefully chosen to allow the liquid to flow across the retina through the retinotomy. Due to the dense posterior vitreous body in rodents, it can act as a physical barrier to the transretinal flow. In our experience, transretinal penetration occurs smoothly when the cannula tip is positioned at approximately 90° to the retina. It is highly recommended, especially at the beginning, to practice using colored solutions, such as fluorescein, to confirm that the injected solution flows only into the subretinal space.
Two limitations of this technique can be identified. First, the transvitreal passage is at risk of touching the lens. A lens touch may be innocuous if the posterior lens capsule is preserved, as it should not lead to cataract formation. However, if the posterior lens capsule is damaged, the risk of cataract formation is high, and the animal may no longer be suitable for the experimental project, particularly if retinal follow-up examinations are required. Second, this technique may lead to bleeding at several stages, which could impede the experimental process. Conjunctival bleeding may result from the eyelid sutures, though this is usually not a problem and can be cleaned with a cotton swab. Additionally, two retinotomies are performed during the procedure, both of which carry a risk of hemorrhage. The first retinotomy is performed while the experimenter is blind to the retina (through the scleral channel), which can lead to intravitreal hemorrhage during scleral perforation, further complicating the procedure. Rarely, subretinal or choroidal bleeding may occur. This 'entry retinotomy' can be avoided by injecting through the cornea9, though this approach significantly limits the range of possible injection sites. The second retinotomy is at a higher risk of bleeding if retinal vessels are touched by the cannula tip. Subretinal hemorrhage must be strictly avoided, as blood components are toxic to photoreceptors10. Because of these risks, novice experimenters should expect a failure rate of 1 in 5 to 1 in 10 injections. In our experience, no cases of choroidal detachment, endophthalmitis, or hypertonia-induced retinal damage have been encountered.
Alternatively, methods using transscleral approaches for subretinal delivery, which avoid transvitreous passage, have been described8,11. However, in our experience, these procedures do not allow for accurate or reproducible injection sites or blebs and should only be performed when using mice. Additionally, these procedures require a self-sealing scleral incision with a needle that crosses the choroid and the retinal pigment epithelium (RPE) at the location of the RD. This poses a risk of subretinal pigment migration, which can lead to inflammatory consequences and may distort the RD model11. Furthermore, the injection is performed without visibility of the syringe tip, increasing the risk of supra- or subchoroidal detachment rather than RD. There is also a risk of unintended retinotomy, leading to subsequent intravitreal delivery, especially when working with retinal degeneration models that have thinner and more fragile retinas.
Applications of subretinal delivery or induced RD include testing various therapeutics on retinal degeneration models or in cases of RD8,11,12,13,14. In both conditions, the goal of the procedure is to mimic processes that ultimately occur in humans. Both transscleral and transvitreous approaches can model RD, as long as the height and duration of the RD are sufficient15. In the case of subretinal delivery, the current methods more closely replicate those used in nonhuman primates16 and humans5, thereby enhancing the translatability of the data.
Ujawnienia
S.P: Consultant and personal financial interests -Pixium Vision, GenSight Biologics (none of these activities are of any relevance to the data presented here).
Podziękowania
This study was supported by the IHU FOReSIGHT (Paris), the Fondation Voir & Entendre (Paris), UNADEV/Aviesan (under the "cone photoreceptor neuroprotection" project, Paris), HyVIS (GA 964468), the Fighting Blindness Foundation (FFB PPA Vision Restoration: PPA-0919-0772-INSERM; FFB PPA Usher 1B: PPA-0922-0840-INSERM). Salaries of A.D. were provided by grants from the Fondation pour la Recherche Médicale (grant number M2R202106013349, Paris), the Fondation de France (grant number WB-2023-49302, Paris) and the French Society of Ophthalmology. The sponsor or funding organizations had no role in the design or conduct of this research.
Materiały
| Name | Company | Catalog Number | Comments |
| Atipemazole (Antidorm 4.27mg/mL) | Axience SAS, Pantin, France | / | Awakening |
| Chloramphenicol-retinol (Ophtalon 10mg/g) | TVM, Lempdes, France | FR/V/3787889 6/1989 | Postoperative care |
| Cover slips Mini 8mm | World Precision Instruments, Sarasota, FL | / | https://www.wpiinc.com/var-1040-cover-slips-pkg-100.html?srsltid=AfmBOoraZfMhuUuY_7rQbM5YKfqM2VR1PT0L-UHQ5uQUdjaeZuogbKP1&utm_source=chatgpt.com |
| Hydroxypropyl methylcellulose | FCI S.A.S., Paris, France | / | Viscous subretinal delivery |
| Ketamine (Ketamidor 100 mg/mL) | Axience SAS, Pantin, France | / | General anesthesia |
| Lumera 700 | Carl Zeiss, Oberkochen, Germany | https://www.zeiss.com/meditec/en/products/surgical-microscopes/ophthalmic-microscopes/opmi-lumera-700.html?utm_source=chatgpt.com | Ophthalmic microscope |
| Medetomidine (Domitor 0.85 mg/mL) | Vetoquinol S.A., Paris, France | https://www.vetoquinol.com/en | General anesthesia |
| Microinjector Micro 4 | World Precision Instruments, Sarasota, FL | SYS-MICRO4 | Injection |
| Oxybuprocaine 1.6 mg/0.4 mL | Théa, Clermont-Ferrand, France | https://www.laboratoires-thea.com/en/chlorhydrate-doxybuprocaine-thea?utm_source=chatgpt.com | Local anesthesia |
| PBS | Life Technologies Europe B.V., Bleiswijk, The Netherlands | 14190-094 | Aqueous subretinal delivery |
| Syringe (10 or 20 µL) | Hamilton, Reno, NV | 80300 | Injection |
| Tear gel (Lubrithal) | Dechron, Shrewsbury, UK | https://www.dechra.ca/our-products/ca/companion-animal/dog/non-prescription/lubrithal?utm_source=chatgpt.com | Cornea protection |
| Tropicamide (Mydriaticum 1 mg/mL) | Théa, Clermont-Ferrand, France | / | Pupil dilation |
Odniesienia
- Guymer, R. H., Campbell, T. G. Age-related macular degeneration. Lancet. 401 (10386), 1459-1472 (2023).
- Hartong, D. T., Berson, E. L., Dryja, T. P. Retinitis pigmentosa. Lancet. 368 (9549), 1795-1809 (2006).
- Cheung, N., Mitchell, P., Wong, T. Y. Diabetic retinopathy. Lancet. 376 (9735), 124-136 (2010).
- Murakami, Y., et al. Photoreceptor cell death and rescue in retinal detachment and degenerations. Prog Retin Eye Res. 37, 114-140 (2013).
- Russell, S., et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet. 390, 849-860 (2017).
- Gagliardi, G., Ben M'Barek, K., Goureau, O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog Retin Eye Res. 71, 1-25 (2019).
- Zeng, R., Zhang, Y., Shi, F., Kong, F. A novel experimental mouse model of retinal detachment: Complete functional and histologic recovery of the retina. Invest Ophthalmol Vis Sci. 53, 1685-1695 (2012).
- Matsumoto, H., Miller, J. W., Vavvas, D. G. Retinal detachment model in rodents by subretinal injection of sodium hyaluronate. J Vis Exp. 79, e50660(2013).
- Timmers, A. M., Zhang, H., Squitieri, A., Gonzalez-Pola, C. Subretinal injections in rodent eyes: Effects on electrophysiology and histology of rat retina. Mol Vis. 7, 131-137 (2001).
- Benner, J. D., Hay, A., Landers, M. B., Hjelmeland, L. M., Morse, L. S. Fibrinolytic-assisted removal of experimental subretinal hemorrhage within 7 days reduces outer retinal degeneration. Ophthalmology. 101, 672-681 (1994).
- Secondi, R., Kong, J., Blonska, A. M., Staurenghi, G., Sparrow, J. R. Fundus autofluorescence findings in a mouse model of retinal detachment. Invest Ophthalmol Vis Sci. 53, 5190-5197 (2012).
- Becker, S., Wang, H., Stoddard, G. J., Hartnett, M. E. Effect of subretinal injection on retinal structure and function in a rat oxygen-induced retinopathy model. Mol Vis. 23, 832-843 (2017).
- Sene, A., Apte, R. S. Inflammation-induced photoreceptor cell death. Adv Exp Med Biol. 1074, 203-208 (2018).
- Zhang, Z. Y., Sun, Y. J., Song, J. Y., Fan, B., Li, G. Y. Experimental models and examination methods of retinal detachment. Brain Res Bull. 169, 51-62 (2021).
- Machemer, R. Experimental retinal detachment in the owl monkey. IV. The reattached retina. Am J Ophthalmol. 66, 1075-1091 (1968).
- Dentel, A., et al. Adaptive optics flood illumination ophthalmoscopy in nonhuman primates: Findings in normal and short-term induced detached retinae. Ophthalmol Sci. 3 (4), 100316(2023).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone