Method Article
Получение встроенной в агаровую бусину Mycobacterium abscessus для инокуляции иммунокомпетентных мышей внутритрахеально
* Эти авторы внесли равный вклад
В этой статье
Резюме
Мыши, как правило, устойчивы к инфекциям, вызываемым Mycobacterium abscessus, что усложняет открытие и разработку столь необходимых антибиотиков против легочных инфекций. Здесь мы описываем метод приготовления посевного материала и внутритрахеальной инфекции, который, как было показано, обеспечивает устойчивую инфекцию у иммунокомпетентных мышей.
Аннотация
Крайне необходимы надежные мышиные модели хронической инфекции Mycobacterium abscessus, патогеном из окружающей среды, который преимущественно инфицирует легкие. Многокомпонентная терапия в течение всего года обеспечивает неприемлемо низкие показатели излечения у пациентов, около 50%, что обуславливает потребность в прогностических доклинических инструментах для разработки более эффективных антибиотиков. Тем не менее, иммунокомпетентные мыши, как правило, устойчивы к легочной инфекции, вызванной M. abscessus. В литературе сообщалось о многочисленных попытках установления устойчивой инфекции M. abscessus у иммунокомпетентных мышей. Среди них наиболее перспективными оказались методы, основанные на бактериях, встроенных в шарики агара, инокулированные мышам C57BL/6 внутритрахеальным путем. Основным ограничением этого подхода является техническая проблема, связанная с получением агаровых шариков воспроизводимого размера и количества бактерий с последующим внутритрахеальным посевом. В этой статье мы сначала подробно опишем оптимизированный протокол, который доставляет нагруженные M. абсцессом шарики агара оптимального диаметра и воспроизводимой бактериальной нагрузки. Далее мы предоставляем подробный протокол внутритрахеального посева, оптимизированный для предотвращения потерь гранул и бактерий из-за прилипания к поверхностям инокуляционных устройств и для достижения воспроизводимого легочного отложения M. abscessus, встроенного в агар. Цель состоит в том, чтобы обеспечить простоту внедрения и превосходную воспроизводимость от лаборатории к лаборатории.
Введение
Варианты лечения легочной болезни Mycobacterium abscessus (Mab) ограничены и включают в себя годичные схемы приема нескольких препаратов с пероральными, парентеральными и иногда ингаляционными антибиотиками, большинство из которых не обладают оптимальной бактерицидной активностью и связаны со значительной токсичностью 1,2,3,4,5 . Срочно необходимы более короткие, безопасные и эффективные методы лечения. Мышиные модели микобактериальных инфекций сыграли важную роль в открытии и оптимизации эффективных лекарств и схем приема лекарств 6,7. Тем не менее, доклиническая оценка антибиотиков против инфекций Mab оказалась сложной задачей из-за трудности установления прогрессирующих и устойчивых легочных инфекций у мышей 8,9.
Инфицирование иммунокомпетентных линий мышей Mab в значительной степени приводит к транзиторной колонизации с последующим быстрым клиренсом возбудителя10,11. Для достижения хронической инфекции, наблюдаемой клинически, иммобилизующие агенты, такие как агароза или агар, были использованы для стимулирования персистенции и иммунопатологического ответа на условно-патогенные микроорганизмы, такие как Pseudomonas aeruginosa или Haemophilus influenza у иммунокомпетентных мышей 12,13,14. Эта модель была недавно адаптирована для достижения хронической респираторной инфекции с помощью Mab ATCC 19977 на иммунокомпетентных мышах C57BL/6N 15,16,17,18. Метод индуцировал персистирующую инфекцию в течение 45 дней после интратрахеальной инокуляции ~1 × 104 до 1 ×10 6 колониеобразующими единицами (КОЕ), с частотой хронической инфекции среди инфицированных мышей 90%-100%19 и образованием организованных гранулем вокруг распадающегося шарика агара18. По-видимому, встраивание Mab в бусины защищает от быстрого выведения врожденной иммунной системой и что бусины, наполненные бактериями, представляют собой очаги для вербовки иммунных клеток, что приводит к образованию гранулем и возникновению хроническойинфекции. Шарики агара/агарозы представляют собой относительно инертный биоматериал, который вызывает минимальные воспалительные реакции. Кроме того, шарики медленно распадаются, что позволяет избежать проблем с аллергическими реакциями на инородные тела или индуцированным фиброзом. Внутритрахеальная инфекция с использованием или без использования устройства для микрораспыления обеспечивает эффективное введение инокулюма в легкие 8,20,21 и воспроизводит клинический контакт и инфекцию лучше, чем внутривенная или интраназальная инокуляция.
Таким образом, модель имеет множество преимуществ. Тем не менее, это также сопряжено с некоторыми проблемами технического характера, а именно: (i) достижение воспроизводимого размера гранул, (ii) получение воспроизводимой бактериальной нагрузки в гранулах и (iii) обеспечение постоянной внутритрахеальной доставки инокулюма. Здесь мы предоставляем подробные протоколы для надежного достижения этих целей и обеспечения воспроизводимости от лаборатории к лаборатории.
протокол
Все исследования на животных проводились в соответствии с Руководством по уходу за лабораторными животными и их использованию Национальных институтов здравоохранения с одобрения Комитета по институциональному уходу за животными и их использованию NIAID (NIH), Бетесда, штат Мэриленд. Все процедуры были одобрены Комитетом по уходу за животными и их использованию в учреждениях. Все исследования с участием M. abscessus проводились в лаборатории с уровнем локализации биобезопасности 2.
1. Приготовление агаровой бусины, внедренной M. abscessus inoculum
- Получить предварительную культуру M. abscessus ATCC 19977 путем инокуляции 1,5 мл культуры оптической плотности (OD600) 1 (с использованием спектрофотометра) в 200 мл среды Middlebrook 7H9 с добавлением 0,05% (v/v) Tween 80, 0,5% (v/v) глицерина и 10% (v/v) обогащения альбумин-декстрозой-каталазой (ADC) Миддлбрука.
- Инкубируйте культуры в роликовой бутылке при температуре 37 °C в течение 24 ч до достижения средней фазы логарифма (наружный диаметр600 = 0,4-0,6).
- Суспензируйте 1,2 г соевого бульона Tryptic (TSB) в 40 мл ультрачистой воды и добавьте 0,6 г агара Difco до конечной концентрации 1,5%.
- Перелейте 60 мл тяжелого минерального масла в колбу Эрленмейера объемом 500 мл и стерилизуйте минеральное масло и триптический соевый агар (TSA) при 121 °C в течение 15 минут, затем уравновесьте при 50 °C в духовке.
- Возьмите образец 1 мл культуры и измерьте наружный диаметр600.
- Соберите клетки центрифугированием при 3700 г в течение 15 мин при 4 °C. Ресуспендируйте клетки в 4 мл 1x фосфатно-солевого буфера Дульбекко (DPBS) для достижения наружного диаметра600 , равного примерно 30.
- Смешайте 3 мл бактериальной суспензии с 27 мл расплавленного TSA, предварительно уравновешенного при 50 °C, в центрифужной пробирке объемом 50 мл. Быстро перемешайте с помощью вортекса или пипетирования.
- Осторожно влейте бактериально-агаровую смесь (30 мл) в 60 мл минерального масла и поместите колбу во вторичную емкость. Быстро установите смесь на среднюю скорость (420 об/мин) на магнитной мешалке, установленной на 50 °C, с помощью магнитной мешалки. Следите за тем, чтобы в масляно-агаровой смеси образовался видимый вихрь. Перемешивайте в течение 6 минут при комнатной температуре (RT).
- Охладите смесь, положив лед во вторичную емкость, и продолжайте помешивать в течение 35 минут.
- Прекратите помешивание и дайте агаровой суспензии отдохнуть в течение 20 минут (при необходимости пополняйте лед).
- Перелейте смесь суспензии в две центрифужные пробирки объемом 50 мл и промойте 10 раз одним объемом DPBS для удаления масла и свободных бактерий (3700 г, 6 мин, 4 °C, в течение первых 5 циклов).
- Соедините суспензии шариков агара в одну трубочку на третьей промывке. Во время первых 5 циклов стирки используйте серологическую пипетку, чтобы аккуратно суспендировать бусины.
- Для последующих 5 циклов стирки используйте следующие параметры центрифугирования: 2000 г в течение 5 минут, затем 1000 г в течение 4, 3, 2 и 2 минут соответственно.
- Суспендируйте шарики агара в DPBS до конечного объема 40 мл, переложите в стерильный флакон объемом 125 мл и разбавьте в 4 раза, добавив 120 мл DPBS.
- Пропустите суспензию через сетчатое фильтр 200 мкм.
- Прикрепите ситечко к стерильной центрифужной пробирке объемом 50 мл. Затем добавьте образец материала на ситечко и отфильтруйте суспензию шариков.
- Чтобы облегчить процесс фильтрации, объедините сетчатое фильтр для клеток, воронку, соединительное кольцо и пробирку объемом 50 мл в одно устройство. Соберите детали в порядке воронки, клеточного фильтра, соединительного кольца и трубки объемом 50 мл и медленно потяните за поршень шприца объемом 30 мл, прикрепленного к соединительному кольцу.
- При необходимости промойте ситечко с противоположной стороны с помощью DPBS, чтобы смыть остатки более крупных шариков. Разбавленную суспензию сконцентрировать, вращая при 1000 г в течение 2 минут или давая шарикам осесть под действием силы тяжести. После сбора урожая бусины из агара хранить при температуре 4 °C до 1 недели. Для каждой партии экспериментов рекомендуется свежая подготовка.
- Пипеткой нанесите 5 л суспензии шарика на предметное стекло, накройте его защитным стеколом и сделайте снимки в нескольких случайных положениях с помощью светового или флуоресцентного микроскопа с объективом 10×. Сохраните изображения и измерьте диаметр валика с помощью ImageJ (желаемый диапазон размеров: 200 ± 50 мкм).
2. Титрование агаровых шариков, внедренных M. abscessus inoculum
- Добавьте 1 мл суспензии шариков в М-пробирку и асептически гомогенизируйте для высвобождения бактерий, встроенных в гранулы, с помощью диссоциатора, установленного на заранее заданную программу RNA.02.01.
- Возьмите 100 мкл гомогената и последовательно разбавьте от 1:10 до 1 × 10-5 разведения. Разбавления на агаре 7H10 с добавлением 10% олеиновой кислоты-альбумин-декстрозы-каталазы (OADC) и 2% глицерина. Перечислите КОЕ через 3-5 дней инкубации.
- При необходимости отрегулируйте титр инокулюма. Например, если каждая мышь получает 100 мкл суспензии в гранулах, содержащей 1 ×10 5 КОЕ, на 10 мышей, всего готовят 2 мл суспензии, содержащей 1 ×10 6 КОЕ/мл, плюс ~25% для учета потерь в шприцах.
3. Тестирование дозирования шариков через шприц и иглу
ПРИМЕЧАНИЕ: Следующие шаги были включены для того, чтобы шарики не прилипли к поверхности шприца или иглы. Было установлено, что важно использовать стеклянный шприц и металлическую подающую иглу 24 Г, чтобы предотвратить потерю бусин из-за прилипания к пластиковым поверхностям.
- Приготовьте PBST, добавив 1,25 мл стерильного Tween от 80 до 500 мл стерильного PBS.
- Добавьте 900 мкл PBST в пять М-пробирок для тестирования шариков агара, проходящих через подающую иглу, а также 900 мкл PBST в 2-мл стерильных пробирок для приготовления от -2 до -6 разведений каждого проталкиваемого образца.
- Смешайте приготовленную ранее бусину с добавлением M. abscessus с помощью пипетки P1000, чтобы убедиться, что все шарики равномерно распределены.
- Прикрепите к стеклянному шприцу металлическую подающую иглу массой 24 г и аспирируйте агаровую суспензию, следя за тем, чтобы не образовывались пузырьки воздуха.
- Как только шприц заполнится без пузырьков воздуха, поместите нейлоновые зажимы или пробки на поршень шприца так, чтобы между зажимами не было места. Пробки обеспечивают точное дозирование 50-100 μл. Есть возможность установить до 6 зажимов вдоль плунжера.
- Выдавите 100 μL шариков («проталкивающих») в пробирки M в соответствующем порядке.
- После того, как будет собрано 5 проталкиваний, гомогенизируйте шарики с помощью диссоциатора тканей, настроенного на настройку РНК.02.01, адекватного для получения РНК из замороженных органов и который, как было обнаружено, эффективно высвобождает M. abscessus из гранул, не влияя на жизнеспособность клеток.
- Выполняйте серийное разведение гомогенатов в диапазоне от -1 до -6. Планшеты для разведения на агаровых пластинах 7H11 с добавлением OADC. Посчитайте КОЕ через 5 дней.
4. Внутритрахеальная инокуляция мышей
- Подготовьте мышей CD-1 в возрасте от 6 до 8 недель.
- Усыпляйте мышей с помощью системы воздействия изофлурана аэрозолем со скоростью потока 1-5%, как описано ранее1.
- Удерживайте мышей с помощью трехмерной (3D) напечатанной наклонной подставки (специально разработанной и изготовленной). Стержень в центре подставки имеет шовную веревку, которая удерживает мышь вертикально за резцы, помещая мышь в брюшное лежачее положение с поднятой головой.
- Прикрепите металлическую иглу для кормления животных 24 г к стеклянному шприцу, наполненному шариком агара, в который вкраплен M. abscessus. Поместите нейлоновые зажимы или пробки на поршень шприца, чтобы заразить каждую мышь 50 μл инокулюма.
- После того, как мышь под действием седативных препаратов будет зафиксирована на шве, переместите язык в сторону рта с помощью аппликатора ватной палочки.
- Визуализируйте заднюю часть рта мыши, чтобы четко определить белую часть в задней части рта и нацелиться на трахею мыши.
- Наклоните металлическую иглу для зонда вертикально над мышью и вставьте ее в верхнюю часть трахеи. При прохождении через надгортанник может наблюдаться небольшое сопротивление.
- Вставьте иглу зонда вниз в трахею, снимите нейлоновый зажим или пробку и выдавите 50 мкл агарового шарика, встроенного в M. abscessus.
- Дайте мышке отдохнуть 5 минут и повторите шаги 4.2-4.8 второй раз. Целью двухэтапной процедуры инокуляции является обеспечение воспроизводимого и глубокого осаждения полного инокулюма в легком.
- Дайте инфекции прогрессировать в течение 24 часов, после чего мышь усыпляют для перечисления отложений бактерий в легких.
Результаты
Размер и нагрузка борта
Чтобы визуализировать агаровые гранулы и их бактериальную нагрузку, красный флуоресцентный белок mCherry экспрессировали под конститутивным промотором Hsp60 в штамме типа Mab ATCC 19977 и встраивали этот штамм в агаровые гранулы, как описано выше. На рисунке 1А показан диапазон размера валика (масштабная линейка = 200 мкм). На рисунке 1B показана нагрузка на 6 независимых препаратов валика, демонстрируя воспроизводимость описанной процедуры.
Нанесение покрытия на шприц, проталкивание и бактериальная имплантация после внутритрахеального посева
Внутритрахеальная инокуляция была оптимизирована в два этапа. КОЕ/мл подсчитывали в инокулюменте до и после прохождения через стеклянный шприц, чтобы гарантировать отсутствие потерь гранул или бактерий при прохождении шариков через шприц и подключенную трубку. В таблице 1 показан начальный бактериальный титр двух независимых препаратов в гранулах (испытания 1 и 2) и количество извлеченных КОЕ на 100 мл «продавленных» образцов из одной загрузки шприца. Чтобы избежать потерь шариков и бактерий, необходим стеклянный шприц, соединенный с металлической трубкой. Бусины агара имеют тенденцию прилипать к пластиковым поверхностям.
Подсчет КОЕ с течением времени в препарате гранул показал, что бактериальная нагрузка гранул оставалась стабильной на уровне 4 °C в течение 1 недели, с ~2-кратной потерей бактериальной нагрузки за 2 недели и ненарушенным отложением в легких. Затем группы от 5 до 8 мышей были инфицированы тремя независимыми препаратами гранул, чтобы проверить воспроизводимость процедуры внутритрахеальной инокуляции. В таблице 2 показано количество КОЕ в легких на одну мышь, перечисленное через 24 часа после заражения, как описано.
Наконец, межоператорная воспроизводимость инфекции оценивалась путем инфицирования трех групп по 10 мышей CD-1, каждая из которых выполнялась разными операторами. КОЕ в легких, перечисленные через 24 ч после заражения, показаны на рисунке 2.

Рисунок 1: Пилотный эксперимент для оценки размера гранул, визуализации и количественного определения бактериальной нагрузки M. abscessus . (A) Бусины агара, содержащие штамм M. abscessus ATCC 19977, сконструированы для экспрессии флуоресцентного белка mCherry red под конститутивным промотором hsp60. Пять μл суспензии в гранулах наносили на предметное стекло и визуализировали с помощью флуоресцентного микроскопа, оснащенного камерой (объектив 10x). Каждая красная точка представляет собой флуоресцентную бактериальную клетку, заключенную в шарики агара диаметром от ~70-250 мкм. (B) Бактериальная нагрузка в КОЕ/мл 6 отдельных препаратов гранул показывает воспроизводимость метода. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этой цифры.
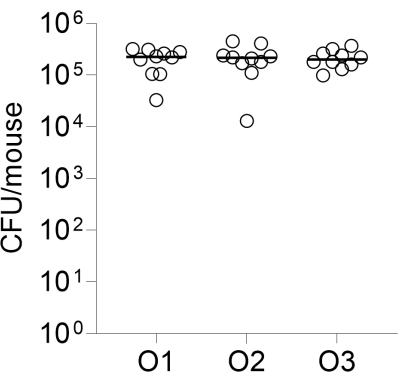
Рисунок 2: Межоператорная воспроизводимость инфекции. Группы из 10 самок мышей CD-1 интрахеально инфицировались тремя независимыми операторами, как описано, с использованием одного и того же препарата в гранулах. Показаны перечисленные КОЕ в легких через 24 ч после заражения. O1, O2, O3: операторы 1, 2 и 3. Пожалуйста, нажмите здесь, чтобы просмотреть увеличенную версию этого рисунка.
| КОЕ/100 μл | ||
| Подготовка швов | Пробное испытание 1 | Испытание 2 |
| 1.13Э+05 | 2.40Э+05 | |
| Образец для проталкивания шприца | ||
| 1 | 1.14Э+05 | 5.10Э+05 |
| 2 | 1.36Э+05 | 4.10Э+05 |
| 3 | 9.30Э+04 | 3.90Э+05 |
| 4 | 9,80Э+04 | 3.60Э+05 |
| 5 | 1.14Э+05 | |
| Средний | 1.11Э+05 | 4.18Э+05 |
| СД | 1.69E+04 (15%) | 6.50E+04 (16%) |
Таблица 1: Колониеобразующие единицы (КОЕ) на 100 μл-образец после прохождения через стеклянный шприц и металлическую подающую иглу.
| Пробное испытание 1 | Испытание 2 | Пробная версия 3 | |||
| Идентификатор мыши | КОЕ в легких/мышь | Идентификатор мыши | КОЕ в легких/мышь | Идентификатор мыши | КОЕ в легких/мышь |
| М-1 | 6.50Э+03 | М-6 | 2.20Э+05 | М-11 | 6.80Э+04 |
| М-2 | 1.10Э+04 | М-7 | 2.70Э+05 | М-12 | 1.70Э+05 |
| М-3 | 1.40Э+04 | М-8 | 8.10Э+04 | М-13 | 2.10Э+05 |
| М-4 | 3.20Э+03 | М-9 | 1.40Э+05 | М-14 | 4.40Э+04 |
| М-5 | 4.50Э+03 | М-10 | 1.30Э+05 | М-15 | 7.50Э+04 |
| М-16 | 1.01Э+05 | ||||
| М-17 | 7.00Э+04 | ||||
| М-18 | 5.80Э+03 | ||||
| Средний | 7.84Э+03 | Средний | 1.68Э+05 | Средний | 9.30Э+04 |
| СД | 4.54E+03 (58%) | СД | 7.57E+04 (45%) | СД | 6.67E+04 (72%) |
Таблица 2: Имплантация шарика агара, внедренного M. abscessus , в легкие мыши через 24 часа после инокуляции
Обсуждение
Чтобы обеспечить равномерное распределение бактерий в шариках агара, важно равномерно взвесить расплавленную смесь агара и бактерий перед добавлением ее в масляную фазу. Объемное соотношение масла и агар-бактерий имеет решающее значение для контроля размера гранул. Более высокое соотношение масла к агару приводит к образованию более мелких шариков агара. При смешивании суспензии агар-бактерий с масляной фазой решающее значение имеет контроль температуры. Масляно-агаровая смесь должна быть достаточно теплой, чтобы обеспечить эмульгирование и образование капель, но достаточно холодной, чтобы не навредить бактериям. Непрерывное перемешивание при охлаждении смеси до комнатной температуры или ниже позволяет каплям агара образовываться и затвердевать.
Правильная калибровка перемешивающей пластины, размер мешалки, а также форма и размер колбы являются важными факторами, определяющими равномерность вихря. Перемешивающая планка, расположенная в центре колбы, создает симметричный вихрь, обеспечивая равномерное перемешивание. Смещенная от центра перемешивание полосы может привести к нестабильному вихрю, в результате чего бусины неправильной формы и появятся пузырьки воздуха в бусинах. Рекомендуется мониторинг и регулировка положения перемешивающей планки в режиме реального времени. Объем колбы должен быть пропорционален объему масляно-агаровой смеси. Колба объемом 500 мл идеально подходит для вращения масляно-агаровой смеси объемом 90 мл, создавая видимый вихрь. При использовании колб большего размера важно регулировать объем масляно-агаровой смеси, размер мешалки и скорость вихря, чтобы достичь баланса между эффективным перемешиванием и стабильным вихрем. Поддержание постоянной скорости завихрения во время процесса и при подготовке сварного шва имеет решающее значение для воспроизводимости от партии к партии.
Процесс приготовления агаровых шариков, в частности, скорость вихревого движения и соотношение масла-агара, адаптированы из16,22 с незначительными изменениями. Поперечные силы внутри вращающегося вихря по своей природе приводят к образованию бусин разных размеров. Чтобы свести к минимуму эту изменчивость размера, стадия сбора бусин была модифицирована путем введения нескольких стадий промывки, используя градиент уменьшающейся скорости центрифугирования и времени для обеспечения удаления мелких гранул (и свободных бактерий). Микросетчатый фильтр был использован для обогащения гранул желаемого размера, эффективно отфильтровывая более крупные гранулы и те, которые могли образоваться в результате случайной коалесценции. Чтобы избежать потерь шариков или бактерий при прохождении суспензии через шприц и подключенную трубку для внутритрахеального инокуляции мыши, крайне важно использовать стеклянный шприц, металлические подающие иглы 24 G и металлическую трубку.
Основным ограничением метода является техническая сложность подготовки шариков и внутритрахеального инокуляции для достижения воспроизводимой нагрузки на шарики и имплантации легких. Требуется внимание к деталям и умение устранять неполадки. Кроме того, модель не была полностью проверена путем тестирования эффективности стандартных антибиотиков и наиболее частых комбинаций антибиотиков, назначаемых пациентам с Маб.
В настоящее время ни одна мышиная модель хронической инфекции Mab не была валидирована до такой степени, чтобы эффективность отдельных агентов и комбинаций лекарств у пациентов можно было обоснованно предсказать с помощью модели9. Мышиная система C57BL/6, встроенная в агаровые шарики, была использована для проверки эффективности антимикробной терапии 15,19,23. Ближайшей альтернативой мышиной системе C57BL/6 со встроенными в бусины агарами является мышиная модель C3HeB/FeJ, которая полагается на иммуносупрессию дексаметазона для достижения длительной хронической инфекции24,25. Преимущества описанного здесь протокола заключаются в том, что (i) можно использовать полностью иммунокомпетентных и наиболее доступных мышей C57BL/6, (ii) продуктивная и хроническая инфекция может быть получена с помощью хорошо охарактеризованного типового штамма ATCC 19977, и (iii) отсутствует потребность в химически индуцированной иммуносупрессии, что позволяет избежать необходимости ежедневных инъекций дексаметазона, тем самым избегая потенциальных лекарственных взаимодействий и влияния на иммунопатологию легких.
Учитывая отсутствие валидированных мышиных моделей хронической инфекции Mab для оценки новых кандидатов в лекарства и схем приема лекарств 8,9, описанный здесь метод может продвинуть эту область вперед, повысить прогностическую ценность данных о доклинической эффективности, помочь определить приоритеты схем лечения с наибольшим потенциалом для обеспечения долгосрочного лечения и в целом ускорить открытие и разработку лекарств для лечения болезни легких Mab. Метод может быть применен к другим патогенам легких и имеет установленный послужной список для P. aeruginosa или Haemophilus influenza. Основная цель описанного здесь протокола заключается в предоставлении достаточных подробностей и рекомендаций для обеспечения простоты реализации и превосходной воспроизводимости от лаборатории к лаборатории.
Раскрытие информации
Авторы заявляют об отсутствии конфликта интересов
Благодарности
Эта работа была профинансирована Фондом муковисцидоза, присуждением DICK24XX0, TD и VD, и R01-AI132374 от NIH-NIAID TD и VD.
Материалы
| Name | Company | Catalog Number | Comments |
| 125-mL bottle | Corning | 8388 | Sterile |
| 3 x 3 Tube Holding Rack | Fisher | 5972-0030PK | Disinfected prior to use |
| 3D Printed Mouse Stand | Transworld Marketing | custom designed and produced | Disinfected prior to use |
| 48-well dilution Plate | Celltreat | 229192 | Sterile |
| 50-mL centrifuge tubes | Greiner Bio-One | 227261 | Sterile |
| Anesethia Machine | E-Z Systems | EZ-AF9000 SYSTEM | n/a |
| Avanti J-15R | Beckman Coulter | B99517 | Centrifuge |
| Barrier Pipette Tips in Lift-off Lid Rack 1000G | Thermo Scientific ART | 21-236-2A | Sterile |
| Biohazard bag | Fisherbrand | 22-044561 | |
| Connector Ring | pluriSelect | 41-50000-03 | |
| Cotton-Tipped Applicators | Puritan | 22-029-571 | Sterile |
| Disposable Inoculation Loops Yellow Sterile 100 in ziplock bag | Cole-Parmer Essentials | 03-391-562 | Sterile |
| Disposable Poly-Lined Towel Drape | Dynarex | 19-310-671 | Sterile |
| Dulbecco's Phosphate-Buffered Saline | Thomas Scientific | 21-031-CM | Sterile |
| Funnel | pluriSelect | 42-50000 | Sterile |
| GE Ultrospec 10 | Sigma-Aldrich | GE80211630 | Spectrophotometer |
| Gentlemacs Tissue Dissociator | Miltenyi Biotec | 130-096-427 | Not heat activated |
| Hamilton Syringe | Hamilton | 80801 | Disinfected prior to use |
| Heavy Mineral Oil | Sigma-Aldrich | 330760-1L | Sterile |
| Isoflurane solution 250 ml bottle | Covetrus | 29405 | Skin Corrosion/Irritation: Category 2, Serious Eye Damage/Eye Irritation: Category 2A, Specific target organ systemic toxicity (single exposure): Category 3 May cause drowsiness and dizziness, Causes serious eye irritation, Causes skin irritation. Do not inhale, use in well ventilated area. |
| M Tubes (gentleMACS) | Miltenyi Biotec | 130-096-335 | Sterile |
| Magnetic stirrer MR Hei-Connect | Heidolph | 505-40000-13-1 | |
| Magnetic stirring bar | Thomas Scientific | 1181L08 | Sterile |
| Mechanical Pipet 100–1000 µL | Gilson PIPETMAN L | FA10006M | Disinfected prior to use |
| Mechanical Pipet 20–200 µL | Gilson PIPETMAN L | FA10003MG | Disinfected prior to use |
| Metal Animal Feeding Needle 24 G | Braintree Scientific | N-VP 24G-1S | Must be sterilized (autoclaved) |
| Middlebrook 7H10 Agar | Sigma-Aldrich | M0303-500G | Sterilized (autoclaved) |
| Middlebrook 7H11 Agar | Thermo Fisher | R4554002 | Sterilized (autoclaved) |
| Middlebrook 7H9 medium | Beckton Dickinson | 271310 | Sterilized (autoclaved) |
| Mouse Stand Parts | TriMech Solutions | SRV-AMS-FDM | Disinfected prior to use |
| Nonabsorbable Silk Suture | Fisher Scientific | 18020-50 | n/a |
| OADC Liquid Enrichment for use with Middlebrook Media 500 ml | Thermo Scientific Remel | R450605 | Sterile |
| PBS (10x), pH 7.4 Sterile Filtered 500 mL | Gibco | 70-011-044 | Sterile |
| Peroxiguard Wipes | Peroxigard | 29221 | n/a |
| Petri Dishes with Clear Lid Round 100mmx 15mm | Fisherbrand | FB0875712 | Sterile |
| PluriStrainer 200 mm | pluriSelect | 43-50200-03 | Sterile; cell strainer |
| Roller bottles | Corning | 430165 | Sterile |
| Serological pipette | Corning | 4489 | Sterile |
| Spectrophotometer cuvettes 1.5 mL | Fisher Scientific | 14955127 | Sterilized (autoclaved) |
| Syringe stoppers or clips (nylon) | Trimech | SRV-AMS-MJF | Disinfected prior to use |
| Tryptic Soy Broth | Sigma-Aldrich | T8907-500G | Sterile |
| Tween 80 | Fisher | 170793 | Filter sterilized |
| Wide Bore Filtered Pipette Tips Lift-off Lid Rack 200G | Thermo Scientific ART | 21-236-1A | Sterile |
Ссылки
- Dartois, V., Dick, T. Drug development challenges in nontuberculous mycobacterial lung disease: Tb to the rescue. J Exp Med. 219 (6), e20220445(2022).
- Martiniano, S. L., Nick, J. A., Daley, C. L. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med. 43 (4), 697-716 (2022).
- Maurer, F. P., et al. Lack of antimicrobial bactericidal activity in mycobacterium abscessus. Antimicrob Agents Chemother. 58 (7), 3828-3836 (2014).
- Wu, M. L., Aziz, D. B., Dartois, V., Dick, T. Ntm drug discovery: Status, gaps and the way forward. Drug Discov Today. 23 (8), 1502-1519 (2018).
- Egorova, A., Jackson, M., Gavrilyuk, V., Makarov, V. Pipeline of anti-mycobacterium abscessus small molecules: Repurposable drugs and promising novel chemical entities. Med Res Rev. 41 (4), 2350-2387 (2021).
- Nuermberger, E. Using animal models to develop new treatments for tuberculosis. Semin Respir Crit Care Med. 29 (5), 542-551 (2008).
- Nuermberger, E. L. Preclinical efficacy testing of new drug candidates. Microbiol Spectr. 5 (3), (2017).
- Nicola, F., Cirillo, D. M., Lore, N. I. Preclinical murine models to study lung infection with mycobacterium abscessus complex. Tuberculosis (Edinb). 138, 102301(2023).
- Dartois, V., et al. Preclinical murine models for the testing of antimicrobials against mycobacterium abscessus pulmonary infections: Current practices and recommendations. Tuberculosis (Edinb). 147, 102503(2024).
- Obregon-Henao, A., et al. Susceptibility of mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother. 59 (11), 6904-6912 (2015).
- Lerat, I., et al. In vivo evaluation of antibiotic activity against mycobacterium abscessus. J Infect Dis. 209 (6), 905-912 (2014).
- Saliu, F., et al. Chronic infection by nontypeable haemophilus influenzae fuels airway inflammation. ERJ Open Res. 7 (1), (2021).
- Rodgers, A. M., et al. Biologically relevant murine models of chronic pseudomonas aeruginosa respiratory infection. Pathogens. 12 (8), 1053(2023).
- Hoover, J. L., et al. A robust pneumonia model in immunocompetent rodents to evaluate antibacterial efficacy against s. Pneumoniae, h. Influenzae, k. Pneumoniae, p. Aeruginosa or a. Baumannii. J Vis Exp. (119), e55068(2017).
- Lore, N. I., et al. The aminoglycoside-modifying enzyme eis2 represents a new potential in vivo target for reducing antimicrobial drug resistance in mycobacterium abscessus complex. Eur Respir J. 60 (6), 2201541(2022).
- Riva, C., et al. A new model of chronic mycobacterium abscessus lung infection in immunocompetent mice. Int J Mol Sci. 21 (18), 6590(2020).
- Chang, V., Phillips, P. P. J., Imperial, M. Z., Nahid, P., Savic, R. M. A comparison of clinical development pathways to advance tuberculosis regimen development. BMC Infect Dis. 22 (1), 920(2022).
- Yang, S. J., et al. Pathological granuloma fibrosis induced by agar-embedded mycobacterium abscessus in c57bl/6jnarl mice. Front Immunol. 14, 1277745(2023).
- Poerio, N., et al. Combined host- and pathogen-directed therapy for the control of mycobacterium abscessus infection. Microbiol Spectr. 10 (1), e0254621(2022).
- Pearce, C., et al. Inhaled tigecycline is effective against mycobacterium abscessus in vitro and in vivo. J Antimicrob Chemother. 75 (7), 1889-1894 (2020).
- De Groote, M. A., et al. Gm-csf knockout mice for preclinical testing of agents with antimicrobial activity against mycobacterium abscessus. J Antimicrob Chemother. 69 (4), 1057-1064 (2014).
- Facchini, M., De Fino, I., Riva, C., Bragonzi, A. Long term chronic pseudomonas aeruginosa airway infection in mice. J Vis Exp. (85), e51019(2014).
- Degiacomi, G., et al. The novel drug candidate vomg kills mycobacterium abscessus and other pathogens by inhibiting cell division. Int J Antimicrob Agents. 64 (4), 107278(2024).
- Rimal, B., et al. T405, a new penem, exhibits in vivo efficacy against m. Abscessus and synergy with beta-lactams imipenem and cefditoren. Antimicrob Agents Chemother. 66 (6), e0053622(2022).
- Maggioncalda, E. C., Story-Roller, E., Ammerman, N. C., Nuermberger, E. L., Lamichhane, G. Progressive mycobacterium abscessus lung infection in c3heb/fej mice associated with corticosteroid administration. bioRxiv. , (2018).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеСмотреть дополнительные статьи
This article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены