Method Article
Mouse Embryonic Lung Culture, A System to Evaluate the Molecular Mechanisms of Branching
Summary
Early embryonic lung organ culture is a very useful system to study epithelial-mesenchymal interactions. Both epithelial and mesenchymal morphogenesis proceeds under specific conditions that can be readily manipulated in this system.
Abstract
Protocol
Organ culture is a very useful and versatile technique to study gene and protein expression. These ex vivo culture methods can be used as preliminary or in complement to in vivo studies.
1. Embryonic Lung Isolation
- Timed-pregnant mice are sacrificed on postcoitum day 11.5 or 12.5 (E11.5-12.5) by CO2 narcosis according to the NIH and OLAW guidelines. The animal is placed in a chamber and 100% CO2 is introduced. After the animal is unconscious the CO2 flow is increased. Clinical death of the animal must be ensured.
- All the following steps need to be completed under sterile conditions in a laminar flow hood. The uterus is removed from the pregnant females. To remove the uterus from the animal, the pregnant females are placed on their back and sprayed with 70% ethanol. An incision is made in the abdomen, and the skin is removed by pulling the skin upwards while holding the animal's hind legs. The peritoneal cavity is opened and the uterus is removed from the animal.
- The blood is rinsed off by placing the uterus in a 50 ml conical tube filled with cold Hanks Balanced Salt Solution (HBSS), and the tube is gently rocked for 2 minutes.
- The uterus is then placed in a Petri dish under the stereoscopic dissecting microscope, and the embryos released from the uterus by incising the uterine wall using spring scissors. The embryos are harvested using a perforated spoon, and placed on ice in a new Petri dish containing HBSS.
- Under the stereoscopic dissecting microscope, embryonic lungs are dissected one by one in a Petri dish containing cold HBSS (Figure 1).
- The isolated embryonic lung is placed on ice in a Petri dish containing HBSS using a sterile transfer pipette.
2. Embryonic Lung Culture
- 500 microliters to 1 milliliter per well of D-MEM/F-12 supplemented with 50 units/ml of Penicillin-Streptomycin is added in a Nunclon Polystyrene dish.
- A Nuclepore Polycarbonate Track-Etch Membrane is placed on top of the media. When the Nuclepore Polycarbonate Track-Etch Membrane is placed on top of the media, make sure that the shiny side is against the media, and rough side is facing upwards.
- A dissected embryonic lung is placed on top of the Nuclepore Polycarbonate Track-Etch Membrane using a sterile transfer pipette.
- The embryonic lung position is adjusted using the forceps. Embryonic lung placed on the filter should have an intact trachea and be placed with their trachea having a straight position, thus allowing all lungs to have the same internal pressure.
- The Nunclon Polystyrene dish is transferred for the desired culture time in a cell incubator (Figure 2).
3. Materials
1. Embryonic Whole Lung Isolation
- Timed-pregnant (C57BL6 wild type or transgenic) mice to be sacrificed on postcoitum day 11.5 to 13.5 (E11.5-13.5).
- Hanks' Balanced Salt Solution (HBSS) (1X), liquid, without calcium chloride, magnesium chloride, or magnesium sulfate. (Invitrogen, Carlsbad, CA) supplemented with 50 units/ml of Penicillin-Streptomycin (Invitrogen, Carlsbad, CA). Store the HBSS at room temperature and at 4°C after opening. Aliquot and store the Penicillin-Streptomycin at -20°C.
- Stereoscopic dissecting microscope (Leica, Wetzlar, Germany).
- Dissection tools including Dumont forceps, Fine iris scissors (straight), Noyes spring scissors and Moria® spoon (perforated) (Fine Science Tools, Foster City, CA).
2. Embryonic Whole Lung Culture
- Dulbecco's Modified Eagle Medium:Nutrient Mix F-12 (D-MEM/F-12) (1X), liquid, 1:1 Contains L-glutamine, but no HEPES buffer. (Invitrogen, Carlsbad, CA) supplemented with 50 units/ml of Penicillin-Streptomycin (Invitrogen, Carlsbad, CA). Keep the DMEM/F-12 bottle away from the light (e.g. covered by aluminum foil) and store at 4°C. Aliquot and store the Penicillin-Streptomycin at -20°C.
- Nuclepore Polycarbonate Track-Etch Membrane, 8.0-m, 13mm (Whatman Nuclepore Track-Etch membrane (Whatman, Florham Park, NJ).
- Nunclon Polystyrene dish with lids, 4 wells (Rochester, NY).
- Disposable transfer pipettes, sterile (Fisher Scientific, Pittsburgh, PA).
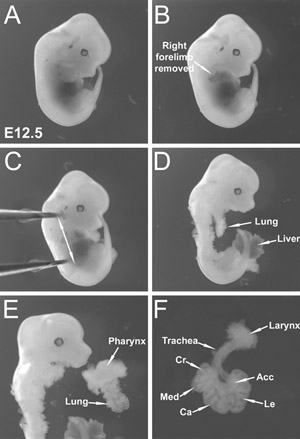
Figure 1. Dissection of E12 embryonic lungs. (A) E12.5 whole embryo viewed from the right side. (B) Right forelimb has been removed from the embryo. (C) Forceps held by the left hand holding the embryo steady in the dish. Arrows indicate plan of dissection (D) Embryonic lung lies posterior to the heart (removed) and anterior to the spine. This figure shows the lungs after skin and heart removal. (E) Pharynx removal allows separation of the intact lung from the embryo. (F) Extraneous pharyngeal tissue and esophagus have been trimmed away. Dissected E12.5 embryonic lung with intact trachea and larynx is shown. (Cr) Cranial lobe, (Med) Medial lobe, (Ca) Caudal lobe, (Acc) Accessory lobe, (Le) Left lobe.
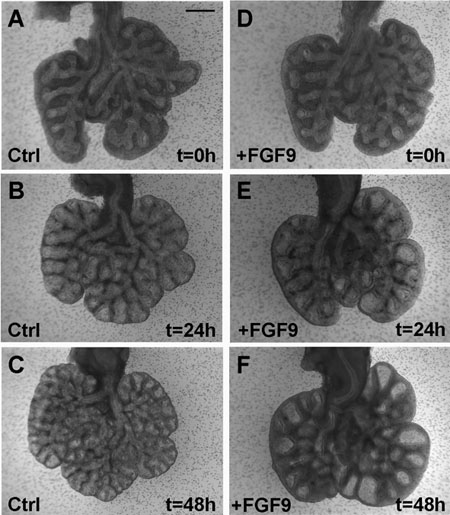
Figure 2. FGF9 induces expansion of the mesenchyme and the dilation of the epithelium in lung grown in vitro. (A-C) E12.5 lung is grown for 48 hours in absence of FGF9. Note the increase in branching. (D-F) E12.5 lung grown for 48 hours in presence of FGF9. Note the dilation of the epithelium and mesenchyme as early as after 24 hours of culture (E). After 48 hours (F), the effect on the epithelium is even more pronounced. Scale bar: A-F 400 μm 4.
The complete text protocol for this experimental approach is available in Springer Protocols.
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by the Saban Research Institute Pre-doctoral Award (PMDM), and by NIH RO1 HL75773 (DW).
References
- Alescio, T., Cassini, A. Induction in vitro of tracheal buds by pulmonary mesenchyme grafted on tracheal epithelium. J Exp Zool. 150, 83-94 (1962).
- Warburton, D., Schwarz, M., Tefft, D., Flores-Delgado, G., Anderson, K. D., Cardoso, W. V. The molecular basis of lung morphogenesis. Mech Dev. 92, 55-81 (2000).
- Spooner, B. S., Hardman, P., Paulsen, A. Gravity in mammalian organ development: differentiation of cultured lung and pancreas rudiments during spaceflight. J Exp Zool. 269, 212-222 (1994).
- Moral, d. e. l., M, P., De Langhe, S. P., Sala, F. G., Veltmaat, J. M., Tefft, D., Wang, K., Warburton, D., Bellusci, S. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev Biol. 293, 77-89 (2006).
- Moral, D. e. l., M, P., Sala, F. G., Tefft, D., Shi, W., Keshet, E., Bellusci, S., Warburton, D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 290, 177-188 (2006).
- Jaskoll, T. F., Don-Wheeler, G., Johnson, R., Slavkin, H. C. Embryonic mouse lung morphogenesis and type II cytodifferentiation in serumless, chemically defined medium using prolonged in vitro cultures. Cell Differ. 24, 105-117 (1988).
- Seth, R., Shum, L., Wu, F., Wuenschell, C., Hall, F. L., Slavkin, H. C., Warburton, D. Role of epidermal growth factor expression in early mouse embryo lung branching morphogenesis in culture: antisense oligodeoxynucleotide inhibitory strategy. Dev Biol. 158, 555-559 (1993).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved