A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Studying Cell Rolling Trajectories on Asymmetric Receptor Patterns
In This Article
Summary
We describe a protocol to observe and analyze cell rolling trajectories on asymmetric receptor-patterned substrates. The resulting data are useful for engineering of receptor-patterned substrates for label-free cell separation and analysis.
Abstract
Lateral displacement of cells orthogonal to a flow stream by rolling on asymmetric receptor patterns presents an opportunity for development of new devices for label-free separation and analysis of cells1. Such devices may use lateral displacement for continuous-flow separation, or receptor patterns that modulate adhesion to distinguish between different cell phenotypes or levels of receptor expression. Understanding the nature of cell rolling trajectories on receptor-patterned substrates is necessary for engineering of the substrates and design of such devices.
Here, we demonstrate a protocol for studying cell rolling trajectories on asymmetric receptor patterns that support cell rolling adhesion2. Well-defined, μm-scale patterns of P-selectin receptors were fabricated using microcontact printing on gold-coated slides that were incorporated in a flow chamber. HL60 cells expressing the PSGL-1 ligand 3were flowed across a field of patterned lines and visualized on an inverted bright field microscope. The cells rolled and tracked along the inclined edges of the patterns, resulting in lateral deflection1. Each cell typically rolled for a certain distance along the pattern edges (defined as the edge tracking length), detached from the edge, and reattached to a downstream pattern. Although this detachment makes it difficult to track the entire trajectory of a cell from entrance to exit in the flow chamber, particle-tracking software was used to analyze and yield the rolling trajectories of the cells during the time when they were moving on a single receptor-patterned line. The trajectories were then examined to obtain distributions of cell rolling velocities and the edge tracking lengths for each cell for different patterns.
This protocol is useful for quantifying cell rolling trajectories on receptor patterns and relating these to engineering parameters such as pattern angle and shear stress. Such data will be useful for design of microfluidic devices for label-free cell separation and analysis.
Protocol
1. HL60 cell rolling
1.1. Fabrication of Patterned Substrates.
- Using microcontact printing (μCP)4-7 to make alternating self-assembled monolayers (SAMs) of PEG molecules on the gold-coated glass slides: Fabricate microcontact printing polydimethylsiloxane (PDMS) stamps that defined the receptor patterns with inclination angle of α = 10° by an SU-8 molding process. Clean the gold surface with piranha solution (3:1 mixture of sulfuric acid to 30% hydrogen peroxide) for 20 minutes and then rinse the surface with copious DI water at 24.5 °C prior to use. Ink the PDMS stamp with 5mM PEG solution in ethanol. Dry the stamp in air for 20 minutes. Gently put the stamp on the gold surface for 40 sec and make sure there is a good contact between the gold surface and the stamp. No excess pressure is required. Rinse the surface with ethanol and dry it under a stream of N2.
- Incubate the substrate within P-selectin solution (15 μg/mL in DPBS) using a perfusion chamber (Electron Microscopy Sciences) for 3 hours at 24.5°C to pattern the remaining areas with P-selectin. Rinse the surface with copious DPBS.
- Backfill the surface with BSA (1 mg/mL in DPBS) for 1 h to block non-specific interactions. Rinse the surface with copious DPBS.
1.2. Cell Rolling Experiments in a Flow Chamber.
- Flow a suspension of HL60 cells (~105 cells/mL) over the patterned surfaces in a rectangular flow chamber (Glycotech, Inc; width w = 1.0 cm; length = 6 cm; height h = 0.0127 cm) at 24.5°C. Use a syringe pump (World Precision Instruments, SP230IW) to generate flow rate of 75 μL/min, with corresponding shear stress around 0.5 dyn/cm2 (~0.05 Pa). Calculate shear stress τ by using the plane Poiseuille flow equation τ = 6μQ/wh2, where μ is the kinematic viscosity (0.001002 Pa s), Q is volumetric flow rate, w is width of the flow chamber, and h is height of the flow chamber.
- Use an inverted microscope (Nikon TE2000-U) with a mounted camera (Andor iXon 885) to record HL60 cells rolling interactions with adhesive P-selectin substrates using a 4× objective, typically at a rate of 1 frame per second for durations of 300 s. Perform three independent experiments, for each shear stress magnitude and pattern inclination angle. Present data as mean and standard deviation of the average values obtained from each experiment.
- Data Analysis.
Analyze the image sequences by a customized Matlab (Mathworks, Inc.) program that utilized a particle tracking freeware8 to generate tracks along the patterned line edges. Tracks extending till the end of a P-selectin band are selected and fitted with two straight line segments - one aligned with the flow, the other aligned with the pattern edge. These two segments are then used to calculate the edge tracking length, rolling velocity on the edge, and rolling velocity on the plain region.
2. Representative Results:
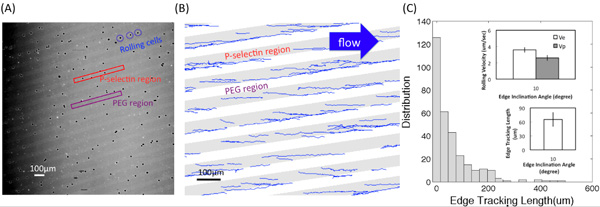
Figure (A) shows one of microscope images converted from the video of HL60 rolling interactions with adhesive P-selectin substrates using a 4× objective. Bright and dark regions correspond to P-selectin receptor and PEG regions, respectively. Figure (B) shows the tracks obtained using a customized Matlab program. The edge inclination angle was 10° and the shear stress was 0.5 dyn/cm2. The edge tracking length, le, displacement, d, and the rolling velocities on the edge and inside the bands, Ve and Vp, respectively, are described in Figure (C-1). Figure (C-2) shows the distribution (the number of recorded cells) of edge tracking length. Insets show the average value of le and the rolling velocity on the edge (Ve) and inside the bands (Vp) at the inclination angle α =10° and the fluid shear stress magnitude around 0.5 dyn/cm2. Error bars represent one standard deviation, where n = 3 replicate experiments (3 replicate surfaces) for each condition.
Discussion
We have described a protocol to examine cell rolling trajectories on asymmetric receptor-patterned surfaces fabricated using microcontact printing2. The optical microscope images of patterned surface showing clear contrast between PEG and P-selectin areas can be used to confirm whether stamping is successful. Sharp, straight edges can be observed when the stamping is performed well. Hard pressing of the stamp may result in stamp deformation which limits the precision of patterns. Wavy edges may be obtained whe...
Disclosures
No conflicts of interest declared.
Acknowledgements
This project was supported by the Deshpande Center for Technological Innovation at MIT (R.K. and J.M.K.) and the NSF CAREER award 0952493 to R.K. through the Chemical and Biological Separations program. We thank the Institute for Soldier Nanotechnologies (ISN) and the Microsystems Technology Laboratory (MTL) at MIT for use of their facilities.
Materials
| Name | Company | Catalog Number | Comments |
| Human promyelocytic leukemia cells | ATCC | CCL-240 | HL60 cells |
| Gold-coated glass slides | EMF | TA134 | Gold slides |
| (1-Mercaptoundec-11-yl)tetra(ethylene glycol) | Sigma-Aldrich | 674508 | PEG |
| Recombinant human P-selectin | R&D Systems | ADP3-050 | P-selectin |
| Bovine serum albumin | Rockland Immunochemicals | BSA-50 | BSA |
| Dulbecco’s phosphate buffered saline | Mediatech, Inc. | 21-030 | DPBS |
| Sulfuric acid | Sigma-Aldrich | 339741 | |
| Hydrogen peroxide | Sigma-Aldrich | 316989 |
References
- Karnik, R., Hong, S., Zhang, H., Mei, Y., Anderson, D. G., Karp, J. M., Langer, R. Nanomechanical control of cell rolling in two dimensions through surface Patterning of receptors. Nano Lett. 8 (4), 1153-1158 (2008).
- Lee, C. H., Bose, S., Van Vliet, K. J., Karp, J. M., Karnik, R. Examining Lateral Displacement of HL60 Cells Rolling on Asymmetric P-selectin Patterns. Langmuir. 27 (1), 240-249 (2011).
- Norman, K. E., Moore, K. L., McEver, R. P., Ley, K. Leukocyte rolling in-vivo is mediated by p-selectin glycoprotein ligand-1. Blood. 86 (12), 4417-4421 (1995).
- Bernard, A., Delamarche, E., Schmid, H., Michel, B., Bosshard, H. R., Biebuyck, H. Printing patterns of proteins. Langmuir. 14 (9), 2225-2229 (1998).
- James, C. D., Davis, R. C., Kam, L., Craighead, H. G., Isaacson, M., Turner, J. N., Shain, W. Patterned protein layers on solid substrates by thin stamp microcontact printing. Langmuir. 14 (4), 741-744 (1998).
- Mrksich, M., Whitesides, G. M. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annual Review of Biophysics and Biomolecular Structure. 25, 55-78 (1996).
- Tan, J. L., Tien, J., Chen, C. S. Microcontact printing of proteins on mixed self-assembled monolayers. Langmuir. 18 (2), 519-523 (2002).
- Lee, D., King, M. R. Microcontact Printing of P-Selectin Increases the Rate of Neutrophil Recruitment Under Shear Flow. Biotechnology Progress. 24 (5), 1052-1059 .
- Greenberg, A. W., Hammer, D. A. Cell separation mediated by differential rolling adhesion. Biotechnol. Bioeng. 73 (2), 111-124 (2001).
- Higuchi, A., Tsukamoto, Y. Cell separation of hepatocytes and fibroblasts through surface-modified polyurethane membranes. J. Biomed. Mater. Res. Part A. 71A (3), 470-479 (2004).
- Alexeev, A., Verberg, R., Balazs, A. C. Patterned surfaces segregate compliant microcapsules. Langmuir. 23 (3), 983-987 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved