A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Shape Memory Polymers for Active Cell Culture
In This Article
Summary
A method for developing cell culture substrates with the ability to change topography during culture is described. The method makes use of smart materials known as shape memory polymers that have the ability to memorize a permanent shape. This concept is adaptable to a wide range of materials and applications.
Abstract
Shape memory polymers (SMPs) are a class of "smart" materials that have the ability to change from a fixed, temporary shape to a pre-determined permanent shape upon the application of a stimulus such as heat1-5. In a typical shape memory cycle, the SMP is first deformed at an elevated temperature that is higher than its transition temperature, Ttrans [either the melting temperature (Tm) or the glass transition temperature (Tg)]. The deformation is elastic in nature and mainly leads to a reduction in conformational entropy of the constituent network chains (following the rubber elasticity theory). The deformed SMP is then cooled to a temperature below its Ttrans while maintaining the external strain or stress constant. During cooling, the material transitions to a more rigid state (semi-crystalline or glassy), which kinetically traps or "freezes" the material in this low-entropy state leading to macroscopic shape fixing. Shape recovery is triggered by continuously heating the material through Ttrans under a stress-free (unconstrained) condition. By allowing the network chains (with regained mobility) to relax to their thermodynamically favored, maximal-entropy state, the material changes from the temporary shape to the permanent shape.
Cells are capable of surveying the mechanical properties of their surrounding environment6. The mechanisms through which mechanical interactions between cells and their physical environment control cell behavior are areas of active research. Substrates of defined topography have emerged as powerful tools in the investigation of these mechanisms. Mesoscale, microscale, and nanoscale patterns of substrate topography have been shown to direct cell alignment, cell adhesion, and cell traction forces7-14. These findings have underscored the potential for substrate topography to control and assay the mechanical interactions between cells and their physical environment during cell culture, but the substrates used to date have generally been passive and could not be programmed to change significantly during culture. This physical stasis has limited the potential of topographic substrates to control cells in culture.
Here, active cell culture (ACC) SMP substrates are introduced that employ surface shape memory to provide programmed control of substrate topography and deformation. These substrates demonstrate the ability to transition from a temporary grooved topography to a second, nearly flat memorized topography. This change in topography can be used to control cell behavior under standard cell culture conditions.
Protocol
1. Isothermal UV-Curing of NOA63
- A custom curing chamber was developed using a glass slide (75 mm x 25 mm x 1 mm), a 1 mm thick Teflon spacer, and an aluminum plate (75 mm x 25 mm x 3 mm) as shown in Figure 1. The chamber is held together using small binder clips.
- Inject the NOA63 into the chamber through a hole in the Teflon spacer using an 18 gauge needle. The NOA63 can be gently heated to ease injection.
- Place the chamber on a hot plate set at 125 °C and allow to heat to a uniform temperature for 5 min.
- Pre-cure the NOA63 in a UV lamp chamber (λmax = 365 nm; see Table) for 20 min with the lamp 6.5 cm from the surface of the glass.
- Remove the NOA63 from the chamber while warm using a razor blade.
- Post-cure the NOA63 under the UV light for 3 h 40 m on the hot plate at 125 °C.
- Store the NOA63 desiccated at -20 °C.
2. Shape Memory Characterization of NOA63
- Prepare a dumbbell specimen by hot-pressing a cured NOA63 film with a customized punch (see Table), whose dimensions are shown in Figure 2.
- Load the specimen into a dynamic mechanical analyzer (DMA; see Table) with tensile fixture. Set the instrument to "force controlled" mode, then program the testing procedure as follows:
- Equilibrate at 95.00 °C
- Isothermal for 10.00 min
- Ramp force 0.300 N/min to 2.500 N
- Isothermal for 5.00 min
- Ramp 2.00 °C/min to 20.00 °C
- Isothermal for 10.00 min
- Ramp force 0.300 N/min to 0.015 N
- Isothermal for 5.00 min
- Ramp 2.00 °C/min to 95.00 °C
- Repeat steps 2-9 two more times
3. Preparation of Active Cell Culture Substrates
- Individual samples can be prepared from the isothermally cured SMP film. Cut the SMP film with a razor to desired sample size. Place the SMP on a hot plate set at a temperature higher than the Tg to reduce the modulus and ease cutting.
- The temporary shape can be fixed in a number of different ways. Here we use a bench top hydraulic press with heated/cooled platens to emboss a temporary topography. Set the temperature of the platens at a temperature above the Tg.
- An embosser was made by curing epoxy on a vinyl record. This will produce a temporary shape of parallel grooves. Here, the embosser had triangular peaks 35 to 40 μm high and 60 μm wide, spaced 80 μm apart. The embosser can be made from other materials and with different topographies, but must be stiffer than NOA63 at the embossing temperature. Place the SMP samples face down on the embosser and place the samples and embosser in the press.
- Apply a ~100 kPa preload to make contact between the heating platens and the samples and hold for ~5 minutes to allow the samples to reach a uniform temperature.
- Apply 1-6 MPa to the samples and hold for 1 minute. A stress of 4.7 MPa leads to incomplete replication of the embosser topography. The temporary topography produced has rounded peaks 25 to 35 μm high and 150 μm wide. The SMP will fracture if a larger stress is applied. Apply smaller stresses to introduce topographies with smaller amplitudes.
- Reduce the temperature below the Tg. Here we use the water cooling capability of the press platens.
- When the temperature is below the Tg, remove the applied force.
- The samples can be stored desiccated at -20 °C. When stored under these conditions, we have observed less than a 1 μm decrease in amplitude recovery after two months for samples embossed with the vinyl record embosser.
4. Active Cell Culture Experiment
- The UV light of a biological safety cabinet (BSC) is used to sterilize the samples. Arrange the samples face down in sterile dishes without lids and turn on the BSC UV light for 6 h.
- Flip samples to new sterile dishes face up. Turn on the BSC UV light for 6 h.
- The samples now need to be equilibrated to a relatively stable state before plating with cells. Place the samples in a 96 well plate and add 150 μl of complete growth medium.
- Put the plate in a 30 °C incubator with 5% CO2 until reaching the desired partial recovery. Here we use 30 h to produce samples with large enough amplitude to align cells.
- The samples can now be plated with cells. Place the samples in a new 96 well plate.
- Add 150 μl of cell solution to the samples. Here we use C3H/10T1/2 mouse embryonic fibroblasts at 20,000 cells/ml to achieve isolated cells (generally not in contact with other cells).
- To allow the cells to attach and spread on the temporary topography, place in a 30 °C incubator for 9.5 h.
- To assay cell morphology before transition, remove samples and perform staining and fluorescence imaging of the samples. This material exhibits autofluorescence through most of the UV and visible range. Fluorophores in the far red end of the spectrum such as Alexa Fluor 647 are recommended to reduce background.
- To trigger samples to recover, move the plate to a 37 °C incubator and continue culture for 19 h to allow the material to recover and allow the cells to adapt their morphology to the new topography.
- Remove samples and apply appropriate stain (phalloidin for filamentous actin imaging) and imaging procedures.
5. Representative Results:
Cured NOA63 is a transparent, glassy solid that has excellent shape memory properties as shown in Figure 3. In this case the material was cured as in Protocol 1 above and shows a uniform Tg of 51.1 °C (determined from the onset of E' drop). It is observed from the one way shape memory cycles (heating, deforming, cooling, recovering, Figure 3) that, a large percentage of strain was fixed after unloading at 20 °C, corresponding to a fixing ratio15 (Rf) of 89.3% (averaged over three cycles; the same below for Rr). The fixed strain recovered at a recovery ratio (Rr) of 84.4% in a relatively small temperature range during heating. Furthermore, the shape memory performance showed no deterioration up to three cycles, in that all the curves follow almost exactly with each other.
NOA63 was used in this protocol because it is readily available from the manufacturer and supplied as an easily cured solvent-free prepolymer with photoinitiator. However, its composition is not disclosed by the supplier. It was found to allow high cell attachment and viability. Finally, the transition temperature could be tuned to allow a meaningful magnitude of recovery between two cell compatible temperatures. A number of other polymer systems could also be used with this protocol if the transition temperature is compatible with cell culture and if they promote cell adhesion and viability.
The amplitude of the temporary topography (grooves) decreases over time at 30 °C. By 30 h at 30 °C, the amplitude has been reduced by ~50% (Figure 4, time 0)16. It reduces another 10% over the next 9.5 h. When recovery is triggered by increasing the temperature to 37 °C, the amplitude reduces to 0.5% of the initial amplitude within 9.5 h. For the embosser used and an embossing stress of 4.9 MPa, this corresponds to a functional change of 13 μm grooves to a nearly flat surface.
An example of a cell behavior controlled through the use of active cell culture substrates is a change in cytoskeleton organization. On temporary grooved substrates before recovery is triggered, the actin microfilaments align along the direction of the grooves (Figure 5a)16. After recovery by temperature increase, the microfilaments have reorganized and are randomly oriented. Control samples that have static grooves or a static flat surface do not reorganize after the increase in temperature (Figure 5b,c).
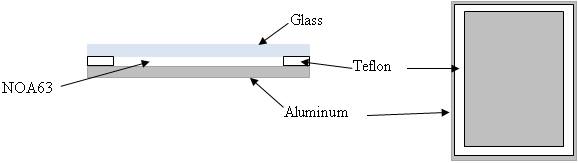
Figure 1: Schematic for NOA63 curing chamber. Cross-section view (left) and top-down view without glass cover (right).
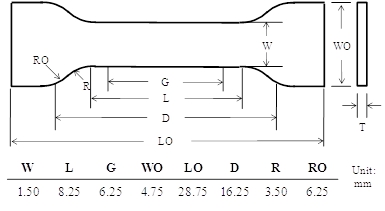
Figure 2: The dumbbell geometry used for bulk shape memory characterization. W: width of narrow section, L: length of narrow section, G: gage length, WO: width overall, LO: length overall, D: distance between grips, R: radius of fillet, and RO: outer radius.
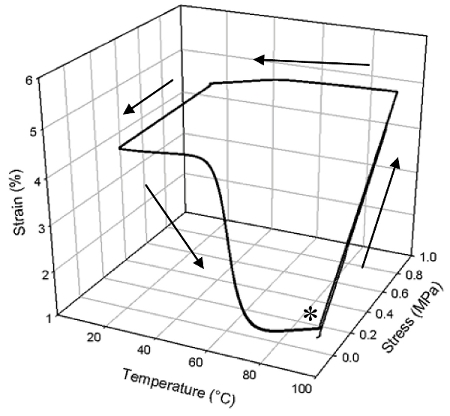
Figure 3: The bulk one-way shape memory of a single NOA63 cure, repeated 3 times (the asterisk indicates experimental onset). At the point denoted with an asterisk, the polymer has been heated and is then deformed by applying a stress to define its temporary shape. This strain is held constant, and the temperature is decreased to fix the temporary shape below the Tg of the polymer. The temperature is then increased, and the material recovers to its permanent shape as the temporary strain is reduced.
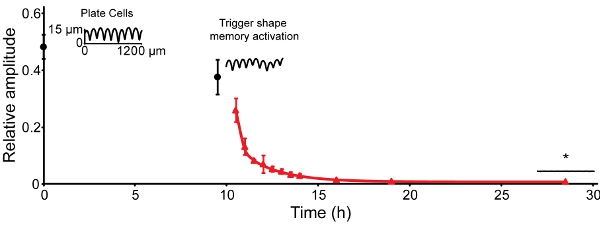
Figure 4: SMP recovery can be triggered under cell-culture-compatible temperatures. The amplitude of 25.6 ± 0.8 μm present following embossing recovered to 12.6 ± 1.5 μm after 30 h equilibration at 30 °C (time 0, black circles). After the samples were moved to a 37 °C incubator (9.5 h), the amplitude recovered to 1.1 ± 0.2 μm within 3.5 h. The amplitude recovered to ~0.3 ± 0.1 μm within 9.5 h and no detectable decrease over the final 9.5 h was observed (red triangles). Error bars represent one standard deviation (n = 4-6). Traces are contact profilometry scans of representative samples.
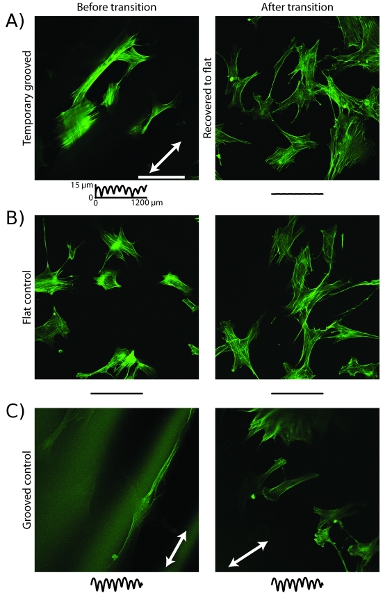
Figure 5: Cell actin cytoskeleton rearranges following topographic transition. a, Confocal images of cells stained with phalloidin on embossed substrates show microfilaments aligned with groove direction (white arrow) before transition and temperature increase. After transition, microfilaments have rearranged are randomly oriented. b, Cells on flat control substrates show randomly oriented microfilaments before and after temperature increase. c, Cells on grooved control substrates show microfilaments aligned with groove direction before and after temperature increase. Scale bar is 100 μm. Traces are as in Figure 4.
Discussion
The Tg of NOA63 can be easily controlled via the curing temperature. We used this to generate SMP substrates that can be triggered in a cell compatible range. NOA63 is plasticized by water which lowers the dry Tg, so we increased the dry Tg by curing at 125 °C to move the wet Tg range between 30 and 37 °C.
The active cell culture substrates demonstrated are able to control cell behavior. The results of microfilament reorganization highlight the potentia...
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors would like to thank Kelly A. Burke for technical assistance with ACC substrate preparation. Based on the article published in Biomaterials, Davis KA, et al., Dynamic cell behavior on shape memory polymer substrates, Biomaterials, doi:10.1016/j.biomaterials.2010.12.006, Copyright Elsevier (2011). This material is based upon work supported by NSF under Grant No. DMR-0907578.
Materials
| Name | Company | Catalog Number | Comments |
| NOA63 | Norland Products, Inc. | NOA63 | Lot number 111 |
| Dogbone Punch | TestResource, Inc. Shakopee, MN | Scaled-down Type IV dogbone (ASTM D638-03) | |
| Benchtop Hydraulic Press | Carver | 3851 | |
| C3H10T1/2 Mouse Embryonic Fibroblasts | ATCC | CCL-226 | |
| Biological Safety Cabinet | Thermo Fisher Scientific, Inc. | 1357 | |
| UV Lamp | Spectroline | SB-100PC | |
| Dynamic Mechanical Analyzer (DMA) | TA Instruments | Q800 | |
| Inverted Fluorescence Microscope | Leica Microsystems | Leica DMI 4000B | |
| Confocal Laser Scanning Microscope | Carl Zeiss, Inc. | LSM 710 | 20x/0.8 NA air or a 40x/1.30 NA oil objective |
References
- Liu, C., Qin, H., Mather, P. T. Review of progress in shape-memory polymers. J. Mater. Chem. 17, 1543-1543 (2007).
- Mather, P. T., Luo, X. F., Rousseau, I. A. Shape Memory Polymer Research. Annu. Rev. Mater. Res. 39, 445-445 (2009).
- Lendlein, A., Kelch, S. Shape Memory Polymers. Angew. Chem. Int. Edit. 41, 2034-2034 (2002).
- Ratna, D., Karger-Kocsis, J. Recent advances in shape memory polymers and composites: a review. J. Mater. Sci. 43, 254-254 (2008).
- Rousseau, I. A. Challenges of shape memory polymers: A review of the progress toward overcoming SMP's limitations. Polym. Eng. Sci. 48, 2075-2075 (2008).
- Pelham, R. J., Wang, Y. L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U. S. A. 94, 13661-13661 (1997).
- Addae-Mensah, K. A., Kassebaum, N. J., Bowers, M. J., Reiserer, R. S., Rosenthal, S. J., Moore, P. E., Wikswo, J. P. A flexible, quantum dot-labeled cantilever post array for studying cellular microforces. Sensor Actuat. a-Phys. 136, 385-385 (2007).
- du Roure, O., Saez, A., Buguin, A., Austin, R. H., Chavrier, P., Siberzan, P., Ladoux, B. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. U. S. A. 102, 2390-2390 (2005).
- Lam, T., Clem, W. C., Takayama, S. Reversible on-demand cell alignment using reconfigurable microtopography. Biomaterials. 29, 1705-1705 (2008).
- Stevens, M. M., George, J. H. Exploring and engineering the cell surface interface. Science. 310, 1135-1135 (2005).
- Tan, L., Tien, J., Pirone, D. M., Gray, D. S., Bhadriraju, K., Chen, C. S. Cells lying on a bed of microneedles: an approach to isolate mechanical. 100, 1484-1484 (2003).
- Teixeira, A. I., Nealey, P. F., Murphy, C. J. Responses of human keratocytes to micro- and nanostructured substrates. J. Biomed. Mater. Res. A. 71A, 369-369 (2004).
- Yang, M., Sniadecki, N., Chen, C. Geometric Considerations of Micro- to Nanoscale Elastomeric Post Arrays to Study Cellular Traction Forces. Adv. Mater. 19, 3119-3119 (2007).
- Zhao, Y., Zhang, X. Cellular Mechanics Study in Cardiac Myocytes Using PDMS Pillars Array. Sensor Actuat. a-Phys. 125, 398-398 (2006).
- DiOrio, A. M., Luo, X., Lee, K. M., Mather, P. T. A Functionally Graded Shape Memory Polymer. Soft Matter. 7, 68-68 (2011).
- Davis, K. A., Burke, K. A., Mather, P. T., Henderson, J. H. Dynamic cell behavior on shape memory polymer substrates. Biomaterials. 32, 2285-2285 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved